Abstract
Background Since 2004, H5N1 outbreaks have been recurrent in domestic poultry and humans in Cambodia. To date, seven human cases (100% CFR) and 22 outbreaks in poultry have been confirmed. Household ownership of backyard poultry (FAO Sector 4 poultry production) in rural Cambodia is high. An understanding of the extent and frequency of poultry handing behaviors in these settings is necessary to assess the risk associated with different practices and to formulate sensible recommendations to mitigate this risk. We collected new data from six geographic regions to examine patterns of human contact with poultry among rural farmers in Cambodia and identify populations with the highest potential exposure to H5N1.
Methods and Findings A cross‐sectional survey was undertaken in which 3,600 backyard poultry owners from 115 randomly selected villages in six provinces throughout Cambodia were interviewed. Using risk assessment methods, patterns of contact with poultry as surrogate measures of exposure to H5N1 were used to generate risk indices of potential H5N1 transmission to different populations in contact with poultry. Estimates of human exposure risk for each study participant (n = 3600) were obtained by multiplying each reported practice with a transmission risk‐weighting factor and summing these over all practices reported by each individual. Exposure risk estimates were then examined stratified by age and gender. Subjects reported high contact with domestic poultry (chickens and ducks) through the daily care and food preparation practices, however contact patterns varied by gender and age. Males between the ages of 26‐40 reported practices of contact with poultry that give rise to the highest H5N1 transmission risk potential, followed closely by males between the ages of 16‐25. Overall, males had a higher exposure risk potential than females across all age groups (p < 0·001).
Conclusions Our results demonstrate that most of the population in rural Cambodia is in frequent contact with domestic poultry. About half of the population in this study carried out on a regular basis at least one of the practices considered to be high risk for the effective transmission if the bird is infected. There was however substantial variation in the frequency of different practices and thus the potential risk of transmission of H5N1 from poultry to humans is not uniform across age and gender even amongst populations living in close proximity to poultry.
Keywords: Animal–human interface, Cambodia, H5N1, risk analysis, semi‐quantitative risk assessment, transmission risk
Background
Since late 2003, highly pathogenic avian influenza (HPAI/H5N1) has spread globally within wild and domestic bird populations and now appears endemic in many parts of Asia and Africa. Millions of people in South‐East Asia and around the world live in close proximity to domestic poultry and although direct contact with infected birds is assumed to be the main source of infection to humans, 1 neither the specific mode of effective transmission from animal to human nor the role of water or other environmental factors 2 is fully understood. Transmission of H5N1 from poultry to human is thought to most likely occur following direct contact with infected poultry organ tissue, blood, nasopharyngeal secretions or faeces under poor hygienic conditions; however, it could also include ingestion of contaminated water. 3 The risk of transmission will be influenced by the nature and frequency of contact with contaminated cells, tissue, blood or secretions in which the virus replicates. 4 , 5 , 6 Most of the H5N1 laboratory‐confirmed human cases to date have reported recent contact with infected poultry although the specific nature of the contact was not recorded. 7 At present there are an excess of reported cases in children and young adults. 8 However, in the absence of detailed exposure data it is not possible to ascertain whether these represent increased exposure, susceptibility to infection, susceptibility to severe disease or a combination of all three. To date three case–control studies have been conducted in Thailand, Vietnam and Cambodia 2 , 9 , 10 to explore risk factors for infection. Exposure from the preparation of sick and dying poultry was noted as an important risk factor in one study 10 but only 38% of the population risk of AI could be attributable to this exposure because of the relatively low prevalence of reporting of this practice. However, the power of these studies is limited because of their small sample size. In addition, there is a lack of reference data on how preparation of sick and dying poultry and other potential exposures differ within and between countries.
Within Cambodia, H5N1 outbreaks have been recurrent since 2004 in domestic poultry and humans. To date, seven human cases, all of which have been fatal, 11 and 22 outbreaks in poultry have been confirmed in villages mainly located in Southern Cambodia. Household ownership of backyard poultry (FAO Sector 4 poultry production) in rural Cambodia is high. 12 An understanding of the extent and frequency of poultry handling behaviours in backyard poultry farming settings is necessary to assess the risk associated with different practices and formulate sensible recommendations to mitigate this risk. Here we present data collected from six geographical regions in Cambodia in which we explore patterns of human contact with poultry among rural farmers to identify populations with the highest H5N1 (or other subtypes of avian influenza) exposure potential.
Methods
Risk assessment framework
A conceptual pathway was developed within the risk assessment framework 13 , 14 and is illustrated in Figure 1. It describes the steps to infection with H5N1 in humans from contact with poultry. The pathway includes the probability that an animal is infected with H5N1 (P), the probability that an individual comes in contact with an infected animal (C), and the probability of effective transmission of H5N1 from poultry to human in the absence of protective clothing (β).
Figure 1.
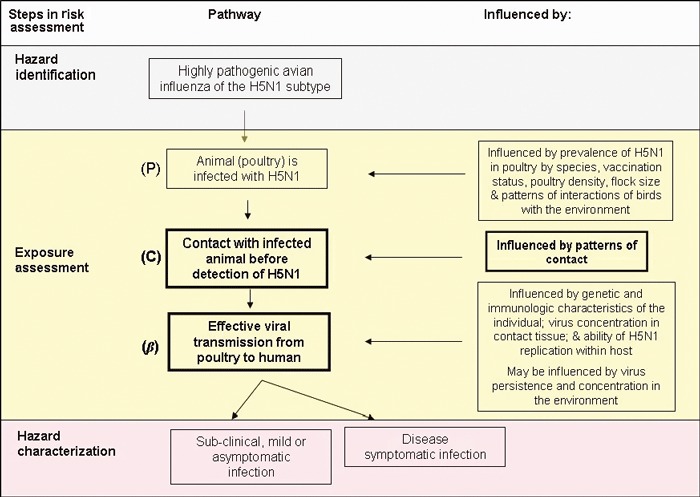
Conceptual pathway for transmission of H5N1 from poultry to humans via contact with poultry.
Several important data gaps and uncertainties currently exist – namely the persistence of H5N1 in domestic/wild poultry populations and in the environment under different atmospheric conditions, virus survival in poultry species during food preparation practices, exposure quantification of H5N1 from poultry and empirical data on risk factors for transmission from poultry to humans – making it difficult to perform a complete quantitative risk assessment. 15 , 16 In this analysis, we contribute new field data to help with such an assessment focusing on the modules outlined in bold (patterns of contact that could result in effective transmission).
Data collection
A cross‐sectional survey was carried out in six provinces using a two‐stage clustered sampling method. 17 Provinces and districts were identified for inclusion in the study from a preliminary assessment of high poultry ownership and human population density; 18 potential cross‐border trading activities, and wild bird mixing (Figure 2). H5N1 has not been suspected nor confirmed in poultry or humans in any of the 115 villages in the study areas; however, it has been confirmed in poultry and humans in one district in Kampong Cham and one district in Prey Veng Province. 7 A random sample of 20 villages per province were selected using probability proportion to population size methodology (village population range 100 to >24 000). 17 Subsequent households were then systematically sampled using a sampling interval having been chosen at random for each village until 30 people [10 male adults (>15 years old), 10 female adults (>15 years old) and 10 children (≤15 years old)] plus one village chief were interviewed. Individuals ≥1 year old, resident in village ≥6 months and medically fit to be interviewed directly or via an adult guardian were included. Ethical approval was granted from the Cambodian Ministry of Health and London School of Hygiene and Tropical Medicine. Prior to sampling, field visits were conducted and meetings were held with provincial veterinarians and village chiefs. Sixteen Cambodian interviewers were trained to administer the questionnaires in Khmer. Informed written consent was obtained from all subjects or their guardians prior to interview.
Figure 2.
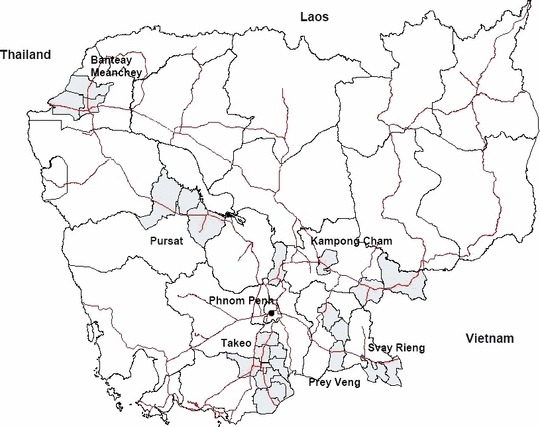
Study areas (districts shaded in grey, national roads indicated in red).
Three separate standardized closed‐ended questionnaires for the head of household, adult family members and children were administered to collect information on the types of direct and indirect contact with domestic and wild poultry. Heads of households were asked about poultry and other animal ownership (quantity of animals owned, husbandry practices, selling/trading practices) while all subjects, including all adults and children, were asked if they had direct contact with domestic poultry through food preparation (slaughter poultry, remove or clean internal organs, cut or wash meat) or other activities (e.g. collect dead domestic/wild poultry for food, eat wild birds, remove feathers from sick poultry, attend fighting cock events), cared for domestic poultry or fighting cocks (feed, clean animals or cages), and in the case of children, played with domestic and/or wild poultry. The nature of how Cambodians prepare poultry for consumption was evaluated by direct observation and informal questioning of adults living in rural Cambodia by the researchers (M.V.K., S.L.) in the field prior to piloting the questionnaires. The questionnaires for all subjects also asked if they had indirect contact with poultry – as a proxy measure of exposure – in the immediate environment around the home and village via water sources (e.g. bathe/swim in ponds where poultry had access). Subjects were asked to recall practices within the previous 8 months, i.e. between the time of the interview and the Khmer New Year Holiday period (April 15). All responses to poultry contact questions were recorded as binary (yes/no) responses and frequencies of contact (when evaluated) were recorded as always, sometimes or never.
Questionnaires were checked daily and discrepancies checked with interviewers/observers prior to double entry into EpiData v3·1 (EpiData Association, Odense, Denmark).
Statistical methods
Prevalence of poultry handling behaviours
Poultry contact patterns were analysed by gender and age using chi‐squared tests or Fisher’s exact tests as appropriate. As a large number of food preparation variables were obtained, principal components analysis (PCA) was used to identify key practices that accounted for the variation observed across the population. Using PCA, a set of eigenvectors and eigenvalues were calculated for each of the factors (i.e. slaughter, boil, remove/wash internal organs, wash/cut meat) describing food preparation. Each principal component is a weighted combination of the original variables. Scree plots 19 were used to retain those components contributing substantially to the overall sample variation. The newly created practice scores created from these principal components were subsequently analysed by gender and age group using t‐tests or Wilcoxon rank‐sum tests as appropriate.
Estimates of human exposure risk
Risk profiles were generated for each subject using their individual poultry handling contact patterns. The probability of effective viral transmission following a certain type of contact is assumed to be high, moderate or low as indicated in Table 1. A transmission risk weighting score (β) was applied to quantify the risk associated with high and moderate practices compared with low practices. Practices listed in group 1 are believed to have a higher potential transmission risk based on the nature of contact and potential H5N1 exposure than practices listed in 2 or 3 whereas practices listed in group 2 have a higher potential transmission risk than practices in group 3. In the analysis presented here, we used values β1 = 10, β2 = 2 and β3 = 1. These values for β1, β2 and β3 are used in this analysis as an illustration of weighting exposures and are based on available data on the pathogenicity of H5N1 in poultry tissues. 3 , 4 , 6 , 20 , 21 , 22 , 23 , 24 , 25 As more epidemiologic and virologic data about the persistence of H5N1 in poultry are collected, more precise estimates for these values may become available. Estimates of human exposure risk for each study participant (n = 3600) were then obtained by multiplying each reported practice with the transmission risk‐weighting factor and summing these over all practices reported by each individual (∑βC). The exposure risks were analysed by age and gender using t‐tests or Wilcoxon rank‐sum tests as appropriate. P‐values of <0·05 were considered statistically significant. All statistical analyses were performed using Stata (v 9·2) (StataCorp, College Station, TX, USA).
Table 1.
Prevalence of practice associated with poultry in rural Cambodian households, main sources of potential exposure and weighted transmission risk potential (β) (n = 3600)
| Probability of effective viral transmission (β grouping) | Practice | Adult males (n = 1201) | Adult females (n = 1199) | Children (n = 1200) | P‐value | Potential viral exposure | |
|---|---|---|---|---|---|---|---|
| >15 years old | ≤15 years old | Adult males versus adult females | Adults versus children | ||||
| High (β1) | Remove internal organs (poultry) | 733 (61·0) | 588 (49·0) | 156 (13·0) | <0·001 | <0·001 | O, B |
| Blow into beak (FC) | 19 (1·6) | 1 (0·1) | 6 (0·5) | <0·001 | 0·27 | NS, B | |
| Kiss, suck, lick wounds (FC) | 10 (0·8) | 0 (0) | 6 (0·5) | 0·002 | 0·72 | B | |
| Share water from the same bottle (FC) | 21 (1·8) | 4 (0·3) | 21 (1·75) | 0·001 | 0·07 | NS, B | |
| Clean trachea (FC) | 44 (3·7) | 1 (0·1) | 16 (1·3) | <0·001 | 0·235 | NS, B | |
| Clean feathers (FC) | 52 (4·3) | 6 (0·5) | 34 (2·8) | <0·001 | 0·46 | B, F | |
| Wash internal organs (poultry) | 745 (62·0) | 775 (64·6) | 249 (20·0) | 0·185 | <0·001 | O, B | |
| Slaughter poultry | 655 (54·5) | 224 (18·7) | 138 (11·5) | <0·001 | <0·001 | B, F | |
| Moderate (β2) | Touch/play with sick poultry or poultry that died from illness | 597 (49·7) | 485 (40·5) | 90 (7·5) | <0·001 | <0·001 | B, F |
| Use poultry faeces as manure | 664 (55·3) | 678 (56·6) | – | 0·534 | – | F | |
| Cut poultry meat | 716 (59·6) | 917 (76·5) | 152 (12·7) | <0·001 | <0·001 | B | |
| Wash poultry meat | 772 (64·3) | 906 (75·6) | 234 (19·5) | <0·001 | <0·001 | B | |
| Swim/bathe in water source where poultry have access* | 56 (14·0) | 41 (10·3) | 196 (16·3) | 0·113 | 0·01 | F, NS | |
| Remove feathers from sick poultry* | 76 (19·0) | 101 (25·3) | 102 (8·5) | 0·04 | 0·001 | NS, B, F | |
| Cleaning/sweeping poultry areas | 843 (70·2) | 903 (75·1) | 442 (36·8) | 0·005 | <0·001 | F | |
| Shopping at wet/live market for poultry | 141 (11·7) | 126 (10·5) | – | 0·341 | – | B, F | |
| Boil poultry | 673 (56·0) | 898 (74·9) | 228 (19·0) | <0·001 | <0·001 | B, F | |
| Low (β3) | Living in a household with poultry (raised chickens or ducks within previous 8 months) | 517 (86·7)** | 1039 (86·6) | – | 0·81 | F | |
Values are expressed as n (%). FC, fighting cocks; B, blood, F, faeces; NS, nasopharyngeal secretions; O, organ tissue; –, not assessed.
*This practice was only evaluated in adults from two provinces (n = 400 adult males and 400 adult females).
**Evaluated from head of household questionnaire only (n = 600).
Results
Poultry handling behaviours of adults and children
A total of 3600 household members [1200 adult (>15 years old) males, adult (>15) females and children (≤15)] were interviewed. The refusal rate was low (<1%). The median age of adult and child subjects was 36 years (range: 16–87) and 9 years (range 1–15), respectively. The prevalence of poultry ownership is high in the study areas with 83·7% of households owning chickens, 35·7% owning ducks and 33·2% owning both chickens and ducks, although most poultry flocks are small [median chicken flock size (interquartile range IQR) = 14 (7–25); duck = 7 (3–15)].
Fighting cock ownership is low (3·8%), whereas ownership of pigs (55%), cattle/water buffalo (63·5%) and dogs (75·5%) is high. Mixing of domestic animals (53·8% of households owned pigs and poultry) is common. In rural areas of Cambodia, chickens and ducks are primarily raised for household consumption. Approximately 11% of adults reported shopping in wet/live markets for poultry. Few households reported selling domestic chickens [3·8% (23/600)] or ducks [0·5% (3/600)] outside their home village or to a market during the previous 8 months. Contact patterns with domestic poultry are provided in Table 1.
Food preparation practices
Preparing poultry for consumption consists of a series of steps including slaughtering the animal by breaking the neck or cutting the throat, bleeding, boiling, defeathering, removing and washing internal organs, and cutting and washing meat. Although family members as young as 2 years old reported that they had prepared poultry for consumption during the study periods, these practices were primarily the responsibility of family members 16–60 years old (Figure 3).
Figure 3.
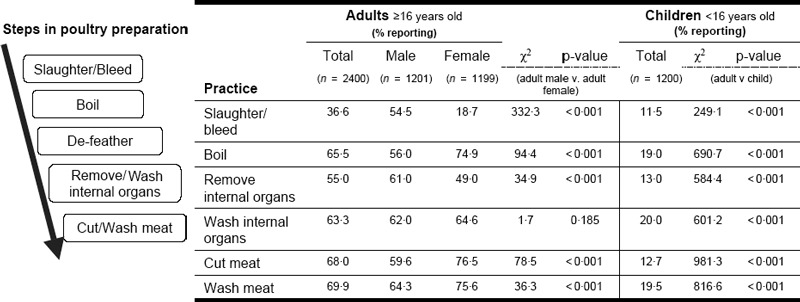
Food preparation practices by age group (N = 3600).
Both men and women were involved in each stage of preparation (Figure 2); however, overall, the proportion of adults involved in all practices related to food preparation was higher than children. Among adults (n = 2400) significantly more men than women slaughter poultry and remove internal organs whereas adult women more often boil poultry, cut meat and wash meat.
Among children, more males than females practice slaughtering (17·0% versus 5·8%, P < 0·001) and removing internal organs (15·7% versus 10·2%, P = 0·005), while more females than males are responsible for boiling poultry (22·3% versus 15·9%, P = 0·005) and cutting meat (15·7% versus 9·8%, P = 0·002).
Principal component analysis of food preparation variables
The first principal component (practice 1), which accounts for approximately 71% of the total variation in practices between individuals in the survey, consisted of all six of the original food preparation variables (boil, slaughter, cut meat, wash meat, remove internal organs, wash organs) and hence can be interpreted as general food preparation. The second principal component (practice 2), which accounts for a further 13% of the variation, was dominated by the practices of slaughtering and removing internal organs.
The frequency of practice 1 (general food preparation, 71% variation) follows a similar age pattern in males and females with the highest scores between the ages of 16–25, 26–40 and 41–60 (Figure 4A). Subjects >60 years old had lower practice scores than children between the ages of 11 and 15 years.
Figure 4.
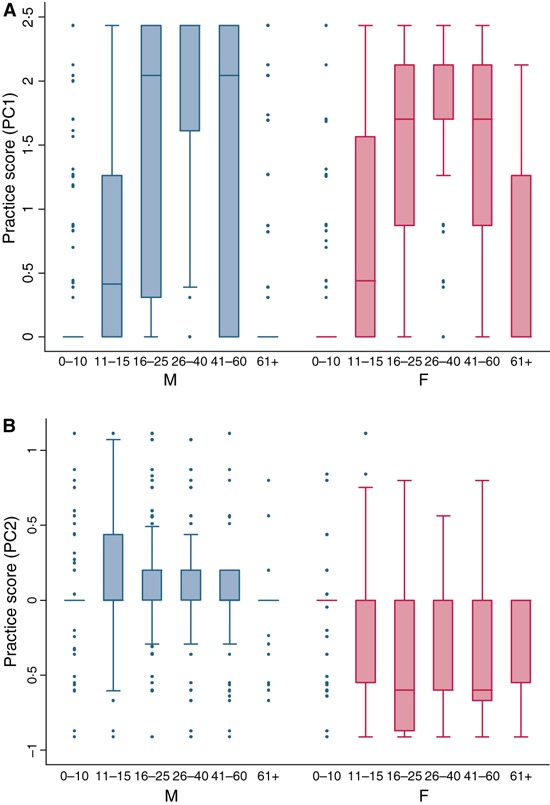
(A) Practice 1 – general food preparation by age and gender. (B) Practice 2 – slaughtering and removing internal organs by age and gender.
Practice 2 (slaughtering and removing internal organs) shows greater differences by gender with this practice predominately undertaken by males (Figure 4B). There are significant differences in practice 2 by gender among subjects with males reporting higher scores than females across all age groupings (two‐sample t‐test P < 0·001).
Other poultry contacts of adults and children
Regular contact with poultry for adult subjects (n = 2400) also includes using faeces for manure (56·6%; no variation by gender), touching sick or dead poultry with bare hands (49·7% in males versus 40·6% in females, P < 0·001), caring for fighting cocks (5·0% in males versus 1·4% in females, P < 0·001), and preparing wild birds for food (36·4% in males versus 19·3% in females, P < 0·001).
Among children (n = 1200) household responsibilities include feeding poultry (77·3%), gathering poultry and placing in designated areas or cages (43·5%), gathering/touching eggs (45·6%), cleaning poultry faeces (44·2% in males versus 37·4% in females, P = 0·02) and treating sick poultry with traditional medicines (18·5%).
Within the recall period, 35·9% of children reported that they had usually played with birds that were alive (42·5% male versus 29·0% female, P < 0·001), 2·7% reported playing with sick birds and 4·2% reported playing with dead birds (no gender difference). Thirty‐two per cent of children removed feathers from sick/dead birds (no gender difference) and 16·3% of children bathed or swam in ponds (no gender difference) in which poultry had access; of those 37·8% reported doing this every day. Twelve per cent of adults (n = 799) reported swimming, bathing or fishing in ponds where poultry have access. Among all subjects who responded to this question (n = 1999), there are no gender differences in reported swimming/bathing in ponds; however, this reported activity was highest in children between the ages of 11 and 15 (16·5%) followed by children between the ages of 1 and 10 (16·2%) compared with adults.
A small number of children were involved in the care of fighting cocks (5·7%; n = 68; Table 1). Among children (n = 1200) 6·7% feed fighting cocks; 2·6% touch bloody fighting cocks; 2·8% clean feathers; 1·3% clean trachea with a swab or feather; 1·8% share water from the same bottle; 0·5% kiss, suck or lick wounds; and 0·5% blow into the beak of a fighting cock (the latter three are practices that occur during fighting cock matches). Twenty‐eight per cent of children reported attending fighting cock matches compared with 11·3% of adults (P < 0·001) (Correction added after publication 20 November 2008: the words ‘adults’ and ‘children’ were inadvertently transposed). Among children, attendance at fighting cock matches was higher among males than females (35·0% versus 20·6%; P < 0·001). Adults reported attending matches on average once per week with the highest proportion of attendance among males between the ages of 16 and 25 years (31·7%).
Estimates of exposure risk
Based on the identified patterns of contact and assumptions of transmission risk (β; Table 1), estimates of exposure risk were calculated for each subject and analysed stratified by age and gender (Figure 5). Overall, the exposure risk was higher among males than females for subjects above the age of 10 (11–15 age group, P = 0·002; 16–25 age group P < 0·001; 26–40 age group, P < 0·001; 41–60 age group, P < 0·001; 61+, P < 0·001). In both males and females exposure risk varies by age with the greatest risks among males between the ages of 26–40 and 16–25 (Figure 5). We also observed a high degree of variability in risk (as seen in the large confidence intervals). Of the 3600 subjects, there were 590 subjects with an exposure risk score above the 90th percentile of the sample. These subjects were predominately male (72·6%) with a median age of 30 (IQR range 21–42).
Figure 5.
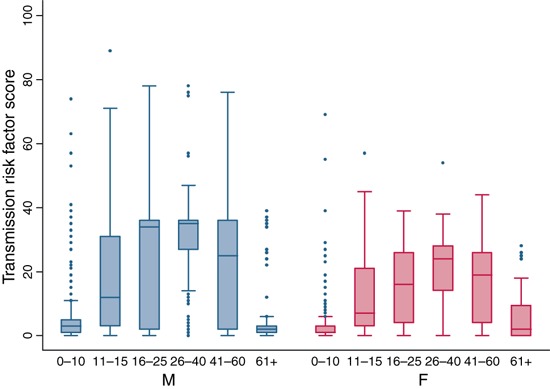
Exposure risk scores by age and gender.
Discussion
Our results demonstrate that most of the population in rural Cambodia is in frequent contact with domestic poultry, with an estimated 52% of the population carrying out on a regular basis at least one of the practices that we considered of high risk of effective transmission if the bird is infected. We also found that the frequency of exposure to poultry was higher in our study population than that reported in the control subjects used in the Vietnamese 10 and Thai 9 case–control studies, suggesting that contact patterns in Cambodia may differ from those in these neighbouring countries. However, at present there are no other similar studies from these countries to enable a direct comparison to be made. Given the widespread exposure to poultry, it is perhaps surprising that only a small number of H5N1 cases have been reported in Cambodia (seven to date). Although there is considerable scope for under‐reporting of human cases the small number of cases may be due to several factors – the lower density of poultry per km2 in Cambodia compared to Thailand and Vietnam, 26 the low probability of people dealing with an infected domestic bird (i.e. low H5N1 prevalence and/or a short duration of infectiousness), and a low probability of effective viral transmission.
Within Cambodia, the typical diet consists primarily of white rice and fish products; animal products compose less than 8% of the daily energy supply. 24 Eating poultry as a source of protein is usually reserved for special occasions, typically weddings and national holidays [e.g. Khmer New Year (April), Chinese New Year (January/February)] and food preparation of poultry therefore differs seasonally.
It is assumed that the probability of risk from preparing and consuming poultry is negligible if food preparation is conducted under strict hygienic conditions. 16 The use of personal protective equipment (i.e. gloves, rubber boots, face masks, aprons) of the subjects in our study areas when in contact with poultry was negligible. Few individuals were in possession of these items in their homes with less than 5% of subjects reported wearing such items when handling poultry. Inactivation of H5N1 on the surface of poultry can occur when the animal is boiled, therefore if poultry are boiled before defeathering as is the case in Cambodia, the risk of exposure during defeathering is reduced. Furthermore, WHO guidelines state that cooking above temperatures of 70°C will inactivate H5N1 in meats and organs, therefore boiling before defeathering would also reduce exposure potential of individuals cutting/washing meat or internal organs. 27
Even though contact was widespread, there was substantial variation in the frequency of different practices, which although differing in magnitude according to practice provided evidence that the potential risk of transmission of H5N1 from poultry to humans is not uniform across age and gender even amongst populations living in close proximity to poultry. Public awareness campaigns and risk behaviour modification intervention programmes should therefore be targeted accordingly.
Males between the age of 26 and 40 reported practices of contact with poultry that give rise to the highest H5N1 transmission risk potential, followed closely by males between the age of 16 and 25. This population group differs from the age and sex distribution of the 357 confirmed H5N1 human cases that occurred up to 29 January 2008, in which an excess of cases were observed in children and no differences observed between genders; however, the group with the highest exposure in our study is more similar to the age/sex distribution of the confirmed Thai cases (n = 25). The mean age of cases was 22 years and 64% of cases were male. 7 Such socio‐demographic differences in human cases of H5N1 may be because contact patterns with poultry differ between countries; however, it is also suggestive that the variation in H5N1 incidence by age may not be due to exposure alone and that there may be differences by age in intrinsic immunologic susceptibility to infection, pre‐existing immunity against human influenza A virus and/or clinical presentation of disease.
This semi‐quantitative risk assessment has several limitations and lacks the power of a formal quantitative risk assessment because of epidemiological data gaps and uncertainties of H5N1 pathogenesis in the host species. To improve future assessments a number of areas would need to be strengthened. First, data are urgently needed on the prevalence of H5N1 in poultry species in regions where H5N1 is recurrent or endemic in domestic poultry flocks. These data are likely to be influenced by the use of biosecurity measures on farms and in backyard farming settings. While H5N1 poultry outbreaks in countries are reported, because infection may remain asymptomatic in some host species (e.g. ducks), it is difficult to infer prevalence from poultry outbreak reports alone. Prevalence estimates in poultry will allow us to fully understand the probability that a farm or animal is infected with H5N1 (P, Figure 1).
Secondly, improved knowledge is needed on all the potential routes of transmission of H5N1 from poultry to humans and the prevalence of such practices in human populations. We have evaluated what we believe are the main potential routes in which people can become infected with H5N1; however, we currently lack sufficient data from the confirmed H5N1 cases around the world to fully evaluate other potential risk factors for infection such as the role water and other environmental factors play in transmission. 28 Transmission could also include oral ingestion, conjunctival or intranasal inoculation from contaminated water while drinking, swimming or bathing or from faeces while caring for poultry 29 and may explain why more children than adults are infected. Furthermore, asymptomatic cases may occur because of low concentrations of viruses in the environment.
Thirdly, an understanding of the influence of genetic and/or immunological factors on transmission is urgently needed since there has been limited yet inefficient human‐to‐human transmission. 30 Lastly, virus transmission potential should not be treated as equal across contact practices. Empirical data are needed on virus survival in poultry during food preparation practices, in poultry waste (i.e. poultry scrap, faeces), in soil and in water under different environmental conditions. In addition, data – either experimentally produced or collected during field investigations – are urgently needed on the persistence of H5N1 in poultry tissues. Specifically, which organ, tissue or secretion, if any, has the greatest potential for poultry‐to‐human transmission. One way of estimating this is to quantify the viral concentrations in various tissues under a variety of conditions (e.g. days post‐infection, whether or not the animal is exhibiting symptoms, by vaccination status, etc.).
Collaboration between human and animal health sectors is essential to understand the risk of transmission between domestic poultry and humans. Current exposure estimates are too general to explain the current pattern or to predict future cases of H5N1 infection in human populations. Rapid, systematic and standardized collection of detailed information on poultry contact patterns in suspected human outbreaks of H5N1 would improve our understanding of transmission from poultry to humans.
Acknowledgements
The authors thank UNICEF for providing the funding for the study. We are very grateful to Sorn San, Vutha Chhim and Nget Kiry from the National Veterinary Research Institute (NaVRI), Department of Animal Health and Production and SreyPech Lim and the Cambodian interviewers for their collaboration in the conducting the survey. We especially thank all the study participants, the Village Chiefs, and District and Provincial Veterinarians for their generous assistance in the survey.
References
- 1. Writing Committee of the Second World Health Organization Consultation on Clinical Aspects of Human Infection with Avian Influenza AV . Update on avian influenza A (H5N1) virus infection in humans. N Engl J Med 2008; 358:261–273. [DOI] [PubMed] [Google Scholar]
- 2. Vong S, Ly S, Sek M, Holl DPB. Environmental contamination during A/H5N1 outbreaks in Cambodia. 2006 Bangkok International Conference on Avian Influenza 2008: Integration from Knowledge to Control, January 23–25, Bangkok, Thailand, 2007.
- 3. WHO . World Health Organization: review of latest available evidence on potential transmission of avian influenza (H5N1) through water and sewage and ways to reduce the risks to human health. World Health Organization, 2006. http://www.who.int/water_sanitation_health/emerging/h5n1background.pdf . [Google Scholar]
- 4. Beato M, Toffan A, De Nardi R, Cristalli A, Terregino C et al. A conventional, inactivated oil emulsion vaccine suppresses shedding and prevents viral meat colonisation in commercial (Pekin) ducks challenged with HPAI H5N1. Vaccine 2007; 25:4064–4072. [DOI] [PubMed] [Google Scholar]
- 5. Isoda N, Sakoda Y, Kishida N, Bai G, Matsuda K et al. Pathogenicity of a highly pathogenic avian influenza virus, A/chicken/Yamaguchi/7/04 (H5N1) in different species of birds and mammals. Arch Virol 2006; 151:1267–1279. [DOI] [PubMed] [Google Scholar]
- 6. Mase M, Tanimura N, Imada T, Okamatsu M, Tsukamoto K et al. Recent H5N1 avian influenza A virus increases rapidly in virulence to mice after a single passage in mice. J Gen Virol 2006; 87:3655–3659. [DOI] [PubMed] [Google Scholar]
- 7. WHO . World Health Organization: avian influenza, 2006–2007. http://www.who.int/csr/disease/avian_influenza/en/ [accessed on 6 June 2008]. [Google Scholar]
- 8. Ji‐Ming C, Ji‐Wang C, Jian‐Jun D, Ying‐Xue S. A survey of human cases of H5N1 avian influenza reported by the WHO before June 2006 for infection control. Am J Infect Control 2007; 35:467. [DOI] [PubMed] [Google Scholar]
- 9. Areechokchai D, Jiraphongsa C, Laosiritaworn Y, Hanshaoworakul W, O’Reilly M et al. Investigation of avian influenza (H5N1) outbreak in humans – Thailand, 2004. MMWR Morb Mortal Wkly Rep 2006; 55:3–6. [PubMed] [Google Scholar]
- 10. Dinh PN, Long HT, Tien NTK, Hien NT, Mai LTQ et al. and the World Health Organization/Global Outbreak Alert and Response Network Avian Influenza Investigation Team in Vietnam . Risk factors for human infection with avian influenza A H5N1, Vietnam, 2004. 2006. http://www.cdc.gov/ncidod/EID/vol12no12/06‐0829.htm .
- 11. Buchy P, Mardya S, Vong S, Toyoda T, Aubin J‐T et al. Influenza A/H5N1 virus infection in humans in Cambodia. J Clin Virol 2007; 39:164–168. [DOI] [PubMed] [Google Scholar]
- 12. Vong S, Goghlan B, Mardy S, Holl D, Seng H et al. Low frequency of avian‐to‐human transmission of H5N1 in Southern Cambodia, 2005. Emerg Infect Dis 2006; 12:1542–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Codex Alimentarius Commission . Principles and Guidelines for the Conduct of Microbiological Risk Assessment. CAC/GL‐30. Rome: Food and Agriculture Organization of the United Nations, 1999. [Google Scholar]
- 14. OIE , ed. Risk Analysis. Paris: World Organization for Animal Health (OIE), 2005. [Google Scholar]
- 15. FAO, OIE, WHO . Food and Agriculture Organization of the Untied Nations, World Organization for Animal Health and the World Health Organization. Technical Workshop on Highly Pathogenic Avian Influenza and Human H5N1 Infection. Proceedings 2 August 2007, Rome, Italy, 2007. [Google Scholar]
- 16. Greiner M, Muller‐Graf C, Hiller P, Schrader C, Gervelmeyer A et al. Expert opinion based modelling of the risk of human infections with H5N1 through the consumption of poultry meat in Germany. Berl Munch Tierarztl Wochenschr Heft 2007; 3/4:98–107. [PubMed] [Google Scholar]
- 17. Bennett S, Woods T, Liyanage W, Smith D. A simplified general method for cluster‐sample surveys of health in developing countries. World Health Stat Q 1991; 44: 98–106. [PubMed] [Google Scholar]
- 18. NIS. National Institute of Statistics . General Population Census of Cambodia, National Institute of Statistics, Ministry of Planning, Phnom Penh, Cambodia. In: National Institute of Statistics C, ed. Phnom Penh, Cambodia, 1999:1–408. [Google Scholar]
- 19. Cattell RB. The scree test for the number of factors. Multivariate Behav Res 1966; 1:245–276. [DOI] [PubMed] [Google Scholar]
- 20. Swayne DE. Understanding the complex pathobiology of high pathogenicity avian influenza viruses in birds. Avian Dis 2007; 51:242–249. [DOI] [PubMed] [Google Scholar]
- 21. Hulse‐Post DJ, Sturm‐Ramirez KM, Humberd J, Seiler P, Govorkova EA et al. Role of domestic ducks in the propagation and biological evolution of highly pathogenic H5N1 influenza viruses in Asia. Proc Natl Acad Sci U S A 2005; 102:10682–10687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Perkins LEL, Swayne DE. Pathobiology of A/Chicken/Hong Kong/220/97 (H5N1) avian influenza virus in seven gallinaceous species. Vet Pathol 2001; 38:149–164. [DOI] [PubMed] [Google Scholar]
- 23. Webster RG, Hulse‐Post DJ, Sturm‐Ramirez KM, Guan Y, Peiris M et al. Changing epidemiology and ecology of highly pathogenic avian H5N1 influenza viruses. Avian Dis 2007; 51:269–272. [DOI] [PubMed] [Google Scholar]
- 24. FAO Nutrition Country Profile – Cambodia. FAO, Rome: Food and Agriculture Organization, 1999:1–24. [Google Scholar]
- 25. Das ASE, Thomas C, Swayne DE, Suarez DL. Detection of H5N1 high‐pathogenicity avian influenza virus in meat and tracheal samples from experimentally infected chickens. Avian Dis 2008; 52:40–48. [DOI] [PubMed] [Google Scholar]
- 26. FAOSTATS 2006–2007 . http://faostat.fao.org/ [accessed on 12 July 2008].
- 27. World Health Organization and International Food Safety Authorities Network (INFOSAN) . Highly Pathogenic H5N1 Avian Influenza Outbreaks in Poultry and in Humans: Food Safety Implications. No. 7/2005 [accessed on 24 April 2008]. [Google Scholar]
- 28. Vong S, Ly S, Sek M, Holl D, Buchy P. Environmental Contamination during Influenza A Virus (H5N1) Outbreaks in Cambodia, 2006. Emerg Infect Dis, 2008; 14:1303–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. De Long MD, Cam BV, Qui PT, et al. Fatal Avian Influenza A (H5Ni) in a Child Presenting with Diarrhea Followed by Coma. Vol. 2005; 352:686–691. [DOI] [PubMed] [Google Scholar]
- 30. Kandun IN, Wibisono H, Sedyaningsih ER, Yusharmen , Hadisoedarsuno W et al. Three Indonesian Clusters of H5N1 Virus Infection in 2005. N Engl J Med 2006; 355:2186–2194. [DOI] [PubMed] [Google Scholar]


