Abstract
The particular bacterium under investigation here (S. pasteurii) is unique in its ability, under the right conditions, to induce the hydrolysis of urea (ureolysis) in naturally occurring environments through secretion of an enzyme urease. This process of ureolysis, through a chain of chemical reactions, leads to the formation of calcium carbonate precipitates. This is known as Microbiologically Induced Calcite Precipitation (MICP). The proper culture protocols for MICP are detailed here. Finally, visualization experiments under different modes of microscopy were performed to understand various aspects of the precipitation process. Techniques like optical microscopy, Scanning Electron Microscopy (SEM) and X-Ray Photo-electron Spectroscopy (XPS) were employed to chemically characterize the end-product. Further, the ability of these precipitates to clog pores inside a natural porous medium was demonstrated through a qualitative experiment where sponge bars were used to mimic a pore-network with a range of length scales. A sponge bar dipped in the culture medium containing the bacterial cells hardens due to the clogging of its pores resulting from the continuous process of chemical precipitation. This hardened sponge bar exhibits superior strength when compared to a control sponge bar which becomes compressed and squeezed under the action of an applied external load, while the hardened bar is able to support the same weight with little deformation.
Keywords: Bioengineering, Issue 110, Microbiologically Induced Calcite Precipitation, Sporosarcinapasteurii, Porous medium, Culture protocol, carbon storage
Introduction
Sporosarcina pasteurii is a gram-positive bacterium able to survive in highly alkaline environments (pH~10)1 and is one of the bacterial species that can become a causative agent of a phenomenon called Microbiologically Induced Calcite Precipitation (MICP)2-4. MICP is a process wherein precipitation of calcium carbonate is induced by certain microbes under suitable environmental conditions. S. pasteurii has assumed importance in recent years due to its identification as a possible agent for inducing significant volumes of MICP under certain conditions. This possibility stems from the fact that S. pasteurii has the unique ability to secrete copious amounts of the enzyme urease. This enzyme acts as a catalyst, promoting an accelerated lysis of urea (a naturally occurring biochemical compound with widespread and abundant supply) in the presence of water molecules. Through a cascade of reactions, this process ultimately leads to the generation of negatively charged carbonate ions. These ions, in turn, react with positive metal ions like calcium to finally form precipitates of calcium carbonate (calcite); hence the label MICP5-9.
The process of MICP has been known and studied for several decades10,11. Over the past few years, MICP has been investigated for a wide range of engineering and environmental applications including bottom-up green construction12, enhancement of large-scale structures13,14 and carbon sequestration and storage15,16.
For example, Cunnigham17 et. al designed a high pressure moderate temperature flow reactor containing a Berea sandstone core. The reactor was inoculated with the bacteria S. fridgidimarina and under conditions of high-pressure supercritical carbon dioxide injection, a massive accumulation of biomass inside the pore volumes was observed, which led to more than 95% reduction in permeability. Jonkers and Schlangen18 studied the effect of certain special strains of bacteria on the self-healing process in concrete. External water transported into the pore network entering through the surface pores is expected to activate the dormant bacteria which in turn help structural strength via MICP. Tobler19 et al. have compared the ureolytic activity of S. pasteurii with an indigenous groundwater ureolytic microcosm under conditions favoring large-scale MCIP and found that S. pasteurii has a consistent capability to improve calcite precipitation even when the indigenous communities lacked prior urease activity. Mortensen20 et.al have studied the effects of external factors like soil type, concentration of ammonium chloride, salinity, oxygen concentration and lysis of cells on MICP. Their demonstration that the biological treatment process is very robust with respect to a wide variation in parameter space substantiates the fitness of this process for various large-scale remediation applications provided a proper enrichment process to reinforce the bacteria is undertaken. Phillips21 et. al designed experiments to study the changes in permeability and strength of a sand column and a sandstone core after being injected with S. pasteurii cultures. They found that while the permeability decreased 2 - 4 times while the fracture strength increased three times.
S. pasteurii and its role in MICP are topics of active research and several issues relating to the mechanism of chemical precipitation are still not fully understood. In light of this, it is very important to have a set of consistent standardized protocols to accurately culture a suitably enriched stock of S. pasteurii to achieve MICP. Here, we outline a rigorous protocol that will ensure repeatability and reproducibility. This manuscript describes the detailed protocols for culturing S. pasteurii and suitably enriching the culture medium to induce precipitation. The process is investigated through various microscopic techniques such as optical and Scanning Electron Microscopy (SEM) and X-Ray Photo-electron Spectroscopy (XPS). The focus of the manuscript is on the process of MICP. Procedures like SEM and SIMS, being well-established standard protocols, are not described separately.
Protocol
NOTE: Perform the experimental protocols in the order described below. The bacterial culture protocol is discussed in Section 1 (Also see Figure 1). Section 2 describes the protocol for enriching the culture medium using external additives. Section 3 describes the protocols for multi-mode microscopy. Weights of all the individual components may be measured using an analytical balance. Volume of each solution may be measured using a volumetric cylinder.
NOTE: Proper biosafety protocols must be followed for Sections 1 - 2. Consult the institutional safety office for details.
1. Bacterial Culture
- Culture Bacteria - Agar Plate Medium Preparation
- Assemble equipment and ingredients such as Petri dishes, flask, Tris-base, HCl, agar, Millipore water, pH-meter etc.Sterilize all containers by autoclaving at 121 °C before use.
- Prepare 1 L of 0.13 M aqueous solution of Tris-buffer by mixing 15.75 g Tris-base with 1 L of Millipore water. To lower the pH level of the original solution (pH 10.4) add 2,800 µl of HCl (50% concentration). Check continuously using a pH-meter to set pH = 9.
- Divide the 1 L buffer solution into two parts as follows:
- Take 800 ml of this solution. Divide it equally into two parts of 400 ml each. Dissolve 8 g (NH4)2SO4 to one solution and 16 g yeast extract to the other solution.
- Take the remaining (200 ml of) solution and divide it again into two parts of 100 ml each. Mix 2 g (NH4)2SO4 to one. Add 4 g yeast extract and 4 g agar to the other.
- Autoclave the 4 solutions separately after wrapping the respective flasks in Al foil and sticking autoclave tapes. NOTE: If a benchtop autoclave unit is used, the volume should be set to 500 ml (temperature and pressure automatically specified as a function of volume).
- After taking them out from the autoclave, set the two 400 ml solutions aside for step 1.3.1 (below). Mix the two 100 ml solutions to have a 200 ml solution. Pour the mixture into 10 - 12 Petri dishes.
- Culture Bacteria - Agar Plate Sample Preparation
- Remove the bacterial stock from freezer (-80 °C) and allow it to thaw. After thawing properly, place the bacterial stock and the agar plate inside a biosafety hood.
- Select the micropipette of smallest available dimension (0.5 - 10 µl is a good choice) to infest the tip with the Sporosarcina pasteurii stock. Streak an agar plate with the micropipette tip. Place the streaked agar plate inside a non-shaking incubator at 31 °C for 48 hr.
- After 48 hr, remove the plate from the incubator and visually examine for the existence of single colonies. If there are no single colonies, then place it in the incubator for another 24 hr.
- Repeat the process until single colonies are detected. Do not exceed 7 days of trial. NOTE: If single colonies do not appear even after a week, then it is concluded that the steps have not been followed properly and the entire process must be repeated from step 1.
- Culture Bacteria - Final Sample Preparation
- Mix the two 400 ml solutions (Tris buffer + (NH4)2SO4 and Tris buffer + yeast extract (1.5.1) together to obtain an 800 ml solution. Transfer 125 ml of this solution into a flask.
- Perform a visual examination of the surface of the agar plate to identify regions with high concentration of single colonies. Gently nudge and break one of the colonies with a micropipette tip.
- Dip the same micropipette tip into the 125 ml flask and stir it thoroughly to ensure that sufficient number of cells for robust multiplication get transferred. Place the flask in a shaking incubator at 150 rpm, 30 °C for 2 - 3 days. After 2 - 3 days, remove the flask from the incubator.
- Culture Bacteria - Final Cell Count
- Perform serial dilution of the non-diluted culture solution using PBS to attain a dilution of at least ten million (10-7) to ensure countable single colonies appear. Draw seven parallel equidistant lines on one of the agar-plates.
- Do this by drawing bold lines on the bottom surface of the Petri dish, prominent enough to be visible from top. Drop 3 little drops of non-diluted solution into one segment. Add 1 ml of non-diluted solution to 9 ml of Phosphate Buffered Saline (PBS) to obtain a 1:10 dilution.
- Take a small aliquot (~ 0.1 ml) of this newly diluted solution with a pipette and drop 3 more small drops on the next segment. Transfer the newly diluted solution to a new flask and further diluted ten times (10x) by adding PBS. This brings down the dilution to 10-2 or 1:100.
- Use this 1:100 solution in the next segment. Repeat this he process with small volumes of the freshly diluted solutions by successively transferring them to new flasks and continuously diluting ten-fold (10x) in tandem with PBS to obtain more and more dilute samples from 10-3 or 1:1,000 all the way down to 10-7 or 1:10 million into the last segment.
- Perform Colony-Forming Unit (CFU) plate count to count the number of cells present in the agar plate after incubating the plate for 1 - 2 days at 31 °C. This gives a quantitative measure of the bacterial count in the undiluted sample. NOTE: The CFU value is measured based on the ability of the system to give rise to colonies under the specific conditions of nutrient medium, temperature and time assuming that every colony is separate and founded by a single viable microbial cell.
- Seal the Petri dishes with self-sealing film and store remaining items in a refrigerator for future use.
2. Protocol for Enrichment of Nutrient to Accelerate Precipitation
Transfer 9 ml of the prepared culture liquid (cells + medium) into several sterilized centrifuge tubes, each of 10 ml volume.
- Prepare a 100 ml stock solution of the external enrichment consisting of four components in the following concentrations in fresh medium: Urea: 2 g/L, Ammonium chloride: 1 g/L, Sodium bicarbonate: 212 mg/L, Calcium chloride: 280 mg/L. Carefully measure all the ingredients using an analytical balance and mix all but urea with fresh medium in a beaker and place in the autoclave (121 °C, 15 psi, 15 min).
- Following autoclave, mix the requisite amount of urea (2 mg) with 1 ml of fresh medium and pass through a syringe fitted with 0.22 µm syringe filter to complete the process of enrichment.
- Add 1 ml of this enrichment medium with additives to the sterilized centrifuge tubes containing 9 ml of the prepared culture liquid (cells + medium) (see step 2.1). NOTE: Of all these components, urea being degradable at elevated temperatures, cannot be sterilized in an autoclave. Hence, after the other components have been autoclaved (121 °C, 15 psi, 15 min), urea is added last through a 0.22 µm syringe filter.
Vortex each tube thoroughly using a mechanical vortexer. Place the enriched liquids (cells + medium + additives) in a non-shaking incubator at 30 °C. Monitor all the units regularly for initiation of precipitation. Using a light microscope, begin microscopic observations once onset of precipitation is detected with the naked eye. This is usually between 30 - 36 hr after start of experiments.
3. Protocol for Multi-mode Microscopy
Transfer a small volume (~ 1 ml) of fluid to a high-magnification microscopy-chambered coverglass system to perform initial observations with bright field mode. To begin with, start all observations with the lowest magnification (4X). which provides a wide area of coverage and hence global information about the distribution of precipitates.
Select particular areas with significant volumes of precipitations and zoom in (20X, 40X, 60X) by progressively increasing the magnification. NOTE: Since it's impossible to properly resolve the cells from the Extracellular Polymeric Substance (EPS) under bright-field (due to very close refractive indices), switch on phase-contrast mode whenever necessary. In this mode, just the outlines of the cell membranes (being a different phase than the bulk cell) are visible, thus enabling visual differentiation.
Finally, stain some chambers with Live/ Dead stain that selectively dyes the live components as green while coloring the dead components in red. Refer to standard protocols28.
Switch on fluorescent mode to properly distinguish between the red and green regions. Red (Red filter cube with Excitation wavelength of 540 - 580 nm, Dichroic mirror of 595 nm and Barrier filter of 600 - 660 nm) and Green (GFP Long-pass Green filter cube with Excitation wavelength of 440 - 480 nm, Dichroic mirror of 505 nm and Barrier filter of 510 nm) filter cubes are used to facilitate fluorescent micrographs.
Once all multi-mode data have been gathered, select the best images and manually overlay (individual brightfield, phase-contrast and fluorescent images) to obtain the best possible contrast and clarity.
Perform SEM on the dried solid samples to understand crystal structures. This is a well-established and standard procedure.22 Perform XPS to identify chemical signatures of functional groups (carbonates). This is a well-established and standard procedure as well.23
Representative Results
S. pasteurii being an alkaliphile24 can survive relatively harsh conditions. When the above mentioned culture protocol is followed, and S. pasteurii is grown inside a chamber, the bacteria leads to the precipitation of calcium carbonate over time (Figure 2A). Figure 2 (b) shows a phase-contrast optical microscopic image of the bacterial cell population within the culture medium. Individual cells can be clearly distinguished, with rod-like shapes characteristic of the bacillus class of bacteria quite evident. Small brown arrows have been used to specifically highlight two individual cells. The precipitation of calcium carbonate occurs over a period of several days. Figure 2A shows how the precipitates settle down at the bottom of a centrifuge tube. This can be vividly seen with the naked eye as a whitish powder sitting below the liquid medium with a sharp contrast in color as opposed to the yellowish liquid. The yellowish liquid contains bacteria suspended in their culture medium. The white precipitates were collected and dried and later imaged using SEM (Figure 2C).
In Figure 3A, the well-defined cleavage lines, a hallmark of crystallinity, can be easily identified. Figure 3B shows the XPS graph for the same sample for characterization. Peaks corresponding to carbonate groups pin-point the existence of calcium carbonate. Thus, Figure 3A and B together conclusively prove the generation of calcite as the precipitated chemical.
A qualitative experiment was performed in order to assess the ability of MICP to change the properties of porous media. To this end, we performed an experiment involving sponges which are expected to mimic a porous medium with a range of length scales of the pore structures, as is found in a typical natural structure. A sponge bar (Mr. Clean, P&G) was immersed in a solution of S. pasteurii for a period of 7 days and then dried. The sponge after long exposure to the MICP process hardened and displayed enhanced compressive strength. This is depicted in Figure 4. The sponge, before immersion (control case), showed significant compression when subjected to a 1 kg weight (Figure 4A - B). On the other hand, after being subjected to immersion in the S. pasteurii solution, it suffered pore clogging due to the resulting calcite precipitation. This led to a massive increase in strength resulting from a bridging of the pore spaces and subsequently enabled the bar to support the 1 kg load without allowing significant compression (Figure 4C - D).
A semi-quantitative idea regarding the hardening process can be gained through measurements of deflection height of the loaded bar. The original sponge sample is a rectangular bar with a height of 4.5 cm. The deflection of the control specimen, very near the point of application of load, is approximately 1.6 cm. In the case of the treated specimen, the deflection reduces to 0.6 cm. Further rigorous quantification of the hardening process is also possible. For example, a mechanistic explanation of this process in terms of increase in shear strength, peak deviatoric stress and dilatancy angles has been proposed by Tagliaferri25 et. al through X-ray imaging and digital image correlation.
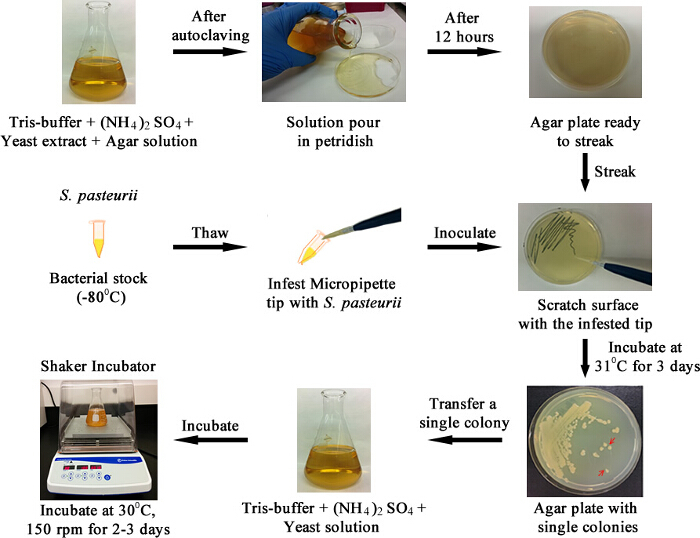 Figure 1. The Outline of the Entire Culture Protocol Represented as an Algorithmic Schematic. To start with, Tris-buffer and agar solution is poured in a Petri dish. Once agar plate is ready, it is streaked with a tip infested with the bacteria. The streaked plate is incubated to grow single colonies. One of the single colonies is transferred to a media flask and incubated again. Please click here to view a larger version of this figure.
Figure 1. The Outline of the Entire Culture Protocol Represented as an Algorithmic Schematic. To start with, Tris-buffer and agar solution is poured in a Petri dish. Once agar plate is ready, it is streaked with a tip infested with the bacteria. The streaked plate is incubated to grow single colonies. One of the single colonies is transferred to a media flask and incubated again. Please click here to view a larger version of this figure.
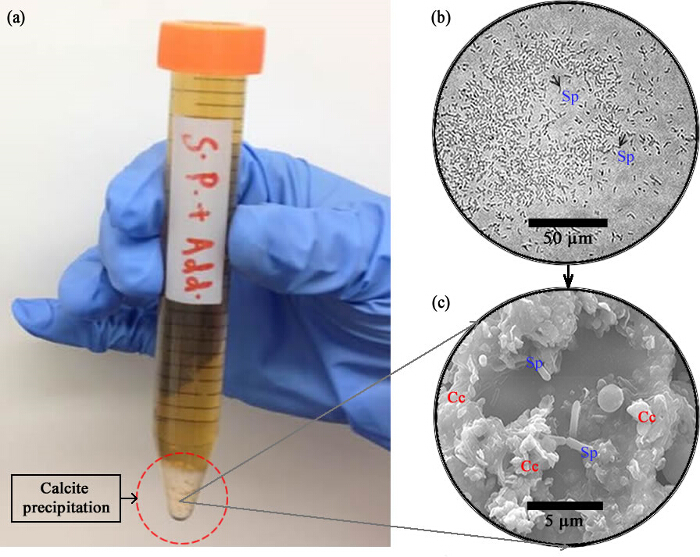 Figure 2. The Cells and the Precipitates. (A) The cells in the culture medium confined inside a centrifuge tube lead to clear observable chemical precipitation (after 5 - 7 days) that settles down at the bottom and is visible to the naked eye. (B) Population of bacteria immobilised on a surface seen under phase-contrast. Individual rod-shaped cells (bacilli, labeled Sp) may be seen clearly (a couple of them highlighted by arrows). (C) SEM image of the white precipitate showing both cells (Sp) and crystals (Cc). Please click here to view a larger version of this figure.
Figure 2. The Cells and the Precipitates. (A) The cells in the culture medium confined inside a centrifuge tube lead to clear observable chemical precipitation (after 5 - 7 days) that settles down at the bottom and is visible to the naked eye. (B) Population of bacteria immobilised on a surface seen under phase-contrast. Individual rod-shaped cells (bacilli, labeled Sp) may be seen clearly (a couple of them highlighted by arrows). (C) SEM image of the white precipitate showing both cells (Sp) and crystals (Cc). Please click here to view a larger version of this figure.
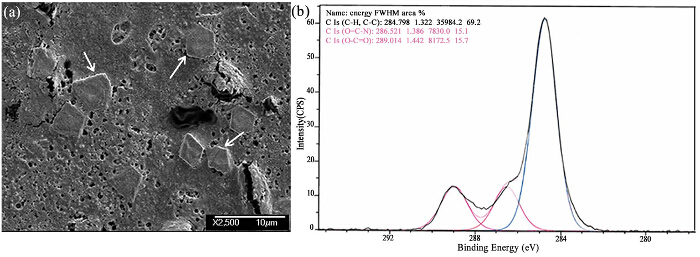 Figure 3. The Crystals and Their Characterization. (A) Scanning electron microscope image of the dried precipitate. Individual crystals and associated cleavage lines are clearly visible. (B) X-Ray Photo-electron Spectroscopy (XPS) graph of the chemical precipitate; mass spectrometry peaks corresponding to carbonate functional group reinforce the possibility of the existence of calcite. Please click here to view a larger version of this figure.
Figure 3. The Crystals and Their Characterization. (A) Scanning electron microscope image of the dried precipitate. Individual crystals and associated cleavage lines are clearly visible. (B) X-Ray Photo-electron Spectroscopy (XPS) graph of the chemical precipitate; mass spectrometry peaks corresponding to carbonate functional group reinforce the possibility of the existence of calcite. Please click here to view a larger version of this figure.
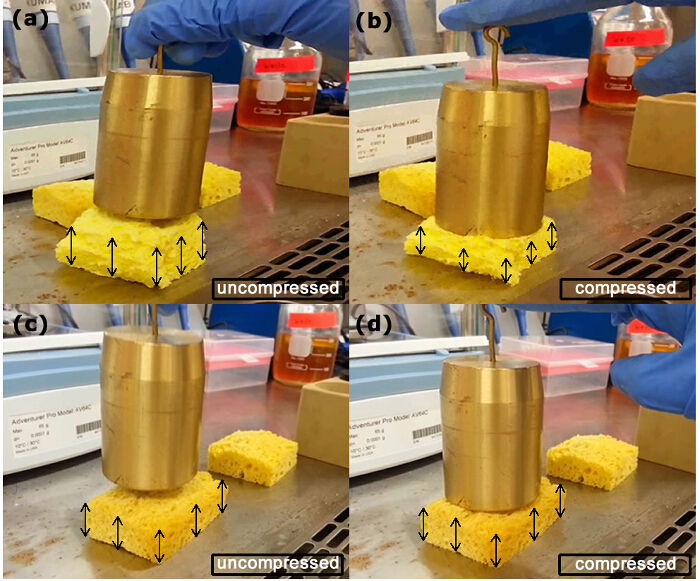 Figure 4. A Semi-quantitative Experiment with Sponge Bars Serving as Prototype Porous Media. (A) and (B) are fresh dry samples not dipped. (C) and (D) are wet samples drenched in a solution of medium and cells; they exhibit a significant increase in material resistance due to pore clogging resulting from calcite precipitation as seen here from reduced compression under the action of a constant weight. Untreated samples are naturally porous and suffer massive reduction in volume when subjected to an 1 kg weight. The pores squeeze out the air and contract, leading to an overall reduction in size. On the other hand, the treated sample has artificially gained a significant amount of stiffness by virtue of pore clogging. It demonstrates a marked enhancement in strength when it comfortably supports the same load without any compression. Please click here to view a larger version of this figure.
Figure 4. A Semi-quantitative Experiment with Sponge Bars Serving as Prototype Porous Media. (A) and (B) are fresh dry samples not dipped. (C) and (D) are wet samples drenched in a solution of medium and cells; they exhibit a significant increase in material resistance due to pore clogging resulting from calcite precipitation as seen here from reduced compression under the action of a constant weight. Untreated samples are naturally porous and suffer massive reduction in volume when subjected to an 1 kg weight. The pores squeeze out the air and contract, leading to an overall reduction in size. On the other hand, the treated sample has artificially gained a significant amount of stiffness by virtue of pore clogging. It demonstrates a marked enhancement in strength when it comfortably supports the same load without any compression. Please click here to view a larger version of this figure.
Discussion
Critical Steps: This manuscript describes in detail the protocols for culturing a viable sample of S. pasteurii. Once the culture has been readied, it must be suitably enriched. This is a key step vital to the success of the experiment because a failure to provide the proper chemical environment leads to either very long time scales of precipitation or a complete lack thereof. S. pasteurii is quite sensitive to several external agencies and must be cultured with a high degree of care and precision to ensure biochemical robustness and repeatability. The extent and dosage of enrichment is now known to dictate the chemical nature of precipitation and hence must be controlled accurately as well.
Modifications/Troubleshooting: Several aspects may be modified with little variance in end-results. The plate count need not be performed per the technique described above (Serial Dilution per the Miles Misra Method). Here, the serial dilution has been performed on a single agar plate by dividing it into seven regions by chords drawn on its bottom surface. This is not mandatory. It's also a common practice to use an entire agar plate for a particular dilution by preparing a spread plate. Serial dilution is performed on a series of successive spread plates and the one with a countable number of single colonies (20 - 200) is chosen for the final calculation. Any of the various other cell counting techniques may be applied here as well. Microscopy may well be performed with simple cover slips or slides. A chamber unit is not at all mandatory.
Sometimes, the bacteria may fail to form precipitates. It is useful to transfer a small amount of the frozen culture into a liquid medium first. Streaking may be performed after growth has occurred in the liquid first.
Limitations: This is a slow technique with a number of steps, one leading to another. There are significant chances of contamination, cross-contamination and other cultural defects at all steps. Intense care must be maintained at all times. Around 180 mg of precipitate is formed per ml of culture solution.
Significance: The present protocol outlines in detail all the steps necessary to ensure that a culture of the bacterium S. pasteurii faithfully precipitates calcite under the influence of externally added enrichments. Although the culture of this bacterium itself is a well-established protocol, the entire process of enrichment and quantification is not. This issue has been addressed here.
Future Applications: It might be possible to suitably modulate this bacterium to precipitate in different manners that in turn can be engineered to various applications depending on the nature of precipitates. It was previously mentioned that there exist several unknowns with respect to MICP involving S. pasteurii. Some of these unknowns include role of aggregative dynamics of S. pasteurii in the MICP process, role of the microscale environment26,27 such as effect of porous media and fluid flow. A standardized protocol can be developed that ensures fast precipitation with a clear opportunity for performing proper multi-mode microscopy.
Disclosures
The authors have nothing to disclose.
Acknowledgments
We wish to acknowledge the partners in the Helmholtz-Alberta Initiative, the Helmholtz Association and the University of Alberta, for the support resulting from participation in this collaboration. Research funding is provided by the Helmholtz Association's Initiative and Networking Fund, the participating Helmholtz Centers and by the Government of Alberta through Alberta Environment's ecoTrust program.
Dr. Tanushree Ghosh is gratefully acknowledged for her critical inputs at a number of crucial stages.
References
- Gibson T. An investigation of the Bacillus pasteurii group. Journal of Bacteriology. 1934;28:491–502. doi: 10.1128/jb.29.5.491-502.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield LJ. Metabolism and concentration of calcium and magnesium and precipitation of calcium carbonate by a marine bacterium. Annals of the New York Academy of Sciences. 1963;109:23–45. [Google Scholar]
- Phillips AJ. Engineered applications of ureolytic biomineralization: a review. Biofouling. 2013;29:715–733. doi: 10.1080/08927014.2013.796550. [DOI] [PubMed] [Google Scholar]
- Dhami NK, et al. Biomineralization of calcium carbonates and their engineered applications: a review. Frontiers in microbiology. 2013;4:314. doi: 10.3389/fmicb.2013.00314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert MO, et al. Controls on the rate of ureolysis and the morphology of carbonate precipitated by S. Pasteurii biofilms and limits due to bacterial encapsulation. Ecological Engineering. 2012;41:32–40. [Google Scholar]
- Okwadha GD, et al. Optimum conditions for microbial carbonate precipitation. Chemosphere. 2010;81:1143–1148. doi: 10.1016/j.chemosphere.2010.09.066. [DOI] [PubMed] [Google Scholar]
- Stocks-Fischer S, et al. Microbiological precipitation of CaCO3. Soil Biology and Biochemistry. 1999;31:1563–1571. [Google Scholar]
- Lauchnor EG, et al. Bacterially induced calcium carbonate precipitation and strontium coprecipitation in a porous media flow system. Environmental science & technology. 2013;47:1557–1564. doi: 10.1021/es304240y. [DOI] [PubMed] [Google Scholar]
- Al Qabany A, et al. Factors Affecting Efficiency of Microbially Induced Calcite Precipitation. Journal of Geotechnical and Geoenvironmental Engineering. 2012;138:992–1001. doi: 10.1061/(asce)gt.1943-5606.0000583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita RY. Calcite precipitation by marine bacteria. Geomicrobiology Journal. 2009;2:63–82. [Google Scholar]
- Chafetz HS. Marine peloids: A product of bacterially induced carbonate precipitation. Journal of Sedimentary Petrology. 1986;56:812–817. [Google Scholar]
- Biomason.com. 2015. Available from: http://www.biomason.com.
- Whiffin VS. Microbial CaCO3 precipitation for the production of biocement. Murdoch University; 2004. PhD thesis. [Google Scholar]
- Paassen LA, et al. Scale up of BioGrout: a biological ground reinforcement method. Proceedings of the 17th International Conference on Soil Mechanics and Geotechnical Engineering. 2009. pp. 2328–2333.
- Cunningham AB, et al. Microbially enhanced geologic containment of sequestered supercritical CO2. Energy Procedia. 2009;1:3245–3252. [Google Scholar]
- Mitchell AC, et al. Biofilm enhanced geologic sequestration of supercritical CO2. International Journal of Greenhouse Gas Control. 2009;3:90–99. [Google Scholar]
- Cunningham AB, et al. Reducing the risk of well bore leakage of CO2 using engineered biomineralization barriers. Energy Procedia. 2011;4:5178–5185. [Google Scholar]
- Jonkers HM, et al. Application of bacteria as self-healing agent for the development of sustainable concrete. Ecological Engineering. 2010;36:230–235. [Google Scholar]
- Tobler DJ, et al. Transport of Sporosarcina pasteurii in sandstone and its significance for subsurface engineering technologies. Applied Geochemistry. 2014;42:38–44. [Google Scholar]
- Mortensen BM, et al. Effects of environmental factors on microbial induced calcium carbonate precipitation. Journal of applied microbiology. 2011;111:338–349. doi: 10.1111/j.1365-2672.2011.05065.x. [DOI] [PubMed] [Google Scholar]
- Phillips AJ, et al. Potential CO2 leakage reduction through biofilm-induced calcium carbonate precipitation. Environmental science & technology. 2013;47:142–149. doi: 10.1021/es301294q. [DOI] [PubMed] [Google Scholar]
- Robbins R. Scanning Electron Microscope Operation. 2010. Available from: http://www.utdallas.edu/~rar011300/SEM/Scanning%20Electron%20Microscope%20Operation.pdf.
- vander Heide P. X-ray Photoelectron Spectroscopy: An introduction to Principles and Practices. John Wiley & Sons; 2011. [Google Scholar]
- Wiley WR, et al. Requirement of an alkaline pH and ammonia for substrate oxidation by Bacillus pasteurii. Journal of Bacteriology. 1962;84 doi: 10.1128/jb.84.4.730-734.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliaferri F, et al. Observing strain localisation processes in bio-cemented sand using x-ray imaging. Granular Matter. 2011;13:247–250. [Google Scholar]
- Kumar A, et al. Microscale confinement features can affect biofilm formation. Microfluidics and Nanofluidics. 2012;14:895–902. [Google Scholar]
- Valiei A, et al. A web of streamers: biofilm formation in a porous microfluidic device. Lab on a chip. 2012;12:5133–5137. doi: 10.1039/c2lc40815e. [DOI] [PubMed] [Google Scholar]
- LIVE/DEAD Bacterial Viability kit, Two-color bacterial viability assay. 2004. https://tools.lifetechnologies.com/content/sfs/manuals/mp07007.pdf, https://www.lifetechnologies.com/ca/en/home/references/protocols/cell-and-tissue-analysis/protocols/live-dead-baclight-bacterial-viability-protocol.html.


