Abstract
Hepatocellular carcinoma (HCC) is a disease with limited treatment options and poor prognosis. In recent years, oncolytic virotherapies have proven themselves to be potentially powerful tools to fight malignancy. Due to the unique dual blood supply in the liver, it is possible to apply therapies locally to orthotopic liver tumors, which are predominantly fed by arterial blood flow. We have previously demonstrated that hepatic arterial delivery of oncolytic viruses results in safe and efficient transduction efficiency of multifocal HCC lesions, resulting in significant prolongation of survival in immune competent rats. This procedure closely mimics the application of transarterial embolization in patients, which is the standard palliative care provided to many HCC patients. The ability to administer tumor therapies through the hepatic artery in rats allows for a highly sophisticated preclinical model for evaluating novel viral vectors under development. Here we describe the detailed protocol for microdissection of the hepatic artery for infusion of oncolytic virus vectors to treat orthotopic HCC.
Keywords: Medicine, Issue 110, Hepatic artery, hepatocellular carcinoma, locoregional therapy, oncolytic virus, transarterial embolization, transarterial chemoembolization, vesicular stomatitis virus, Newcastle disease virus
Introduction
Hepatocellular carcinoma (HCC) is the fifth most prevalent cancer worldwide, and the third leading cause of cancer-related death, making it a significant health concern1,2. For patients who are not eligible for tumor resection, or those awaiting liver transplantation, locoregional therapy involving transarterial embolization (TAE) or transarterial chemoembolization (TACE) are applied as standard palliative care3,4. These therapies exploit the unique feature of dual blood supply in the liver whereby tumors are fed almost exclusively by hepatic arterial blood flow, while the surrounding liver receives the majority of its blood supply from the portal vein5,6.
Due to the extremely limited efficacies of established therapies for HCC, oncolytic viruses have emerged as promising alternative therapeutics. JX-594, recently renamed Pexa-Vec, is a thymidine kinase-deleted vaccinia vector, armed with granulocyte-macrophage colony-stimulating factor (GM-CSF), which has completed phase II clinical trial for HCC7. More recently, a recombinant vesicular stomatitis virus vector (VSV) expressing human interferon-beta has entered a phase I clinical trial for sorafenib-refractory HCC (NCT01628640). As oncolytic viruses move closer to obtaining approval for clinical application for HCC in patients, the need for an effective administration route to target multifocal disease is evident. While systemic delivery is largely ineffective due to inefficient tumor transduction, intratumoral applications could limit the efficacy of the therapy to the injected tumor, leaving uninjectable microscopic lesions susceptible to disease progression.
We have established a method of isolating the hepatic artery in rats to administer oncolytic virus therapy in a locoregional manner to target orthotopic HCC. We have demonstrated that this administration route results in safe and effective transduction of multifocal HCC nodules, resulting in significant survival prolongation in immune competent rats8-10. Here, we describe the method of accessing, dissecting, and injecting into the hepatic artery in rats. A scheme of the procedure is shown in Figure 1 (previously published9)
Protocol
Note: The following steps have been performed in accordance with the guidelines of our institution and the local government of Bavaria, Germany. All attempts to reproduce this protocol must be made in adherence with the local guidelines for the humane treatment of animals, as dictated by local animal care and use committee. For example, many institutes require that sterile gloves are worn during rodent surgery. Furthermore, work with viruses must be performed according to local regulations, taking care for personal and environmental safety. Care must be taken to properly dispose of contaminated waste and to clean instruments and workspace (i.e., by autoclaving and cleaning with an appropriate antiviral disinfectant according to the manufacturer's instructions).
1. Preparations Before Beginning Surgery
Sterilize surgical instruments (i.e., by autoclaving).
- Dilute the virus to the appropriate concentration, which will vary depending on the toxicity of the specific virus being used and the susceptibility of the rat strain. Note: For example, when using vesicular stomatitis virus in male Buffalo rats, we typically inject 107 pfu in a 1 ml volume, which is the maximum tolerated dose in that model.
- Prefill the virus (using aseptic technique) in 1 ml syringes, and attach 30 G needles, taking care to eliminate air from the syringe and needle.
Prepare the surgical space with all materials and equipment required, and spray the area with ethanol to disinfect. Fill a small receptacle with sterile saline solution (0.9% NaCl) for moistening gauze swabs and cotton-tipped applicators.
2. Preparation of the Rat
Administer an analgesic (as specified by the local animal care and use committee), at the appropriate dose 30 min prior to beginning the surgical procedure. For example, administer a high dose of metamizole (100-200 mg/kg).
- Anesthetize the rat using inhalation (isoflurane) or injectable (i.e., a mixture of Medetomidine (0.15 mg/kg), Midazolam (2 mg/kg), and Fentanyl (0.005 mg/kg), also known as MMF) anesthesia. Administer isolflurane through a vaporizer at 1-3% and adjust as necessary to maintain normal respiration and heart rate.
- Before proceeding, ensure that the rat is fully sedated and unable to feel pain by pinching the foot pad and observing for a reaction.
- Apply a veterinary eye ointment to prevent dryness during anesthesia.
Shave the abdominal area from the sternum down to just above the genitals using a small veterinary hair clipper. Take care to achieve a close shave without injuring the skin.
Fix the rat to the surgical bed using tape on the fore- and hindlimbs, and, if using inhalation anesthesia, place the snout into a suitably-sized nose cone. Place a heating pad underneath the rat to maintain body temperature. Disinfect the abdominal area using a surgical grade disinfectant, followed by an alcohol rinse and application of an antiseptic paint.
3. Laparotomy
Using a small curved scalpel, such as #15, make a small vertical incision in the skin layer directly along the midline. Extend the incision up to the xyphoid process. Next, incise the muscle layer by gently lifting up with a pair of Adson forceps to make a scalpel incision, and then extend the incision up to the xyphoid process with surgical scissors. Take care not to puncture the organs of the abdominal cavity or to damage the small vessels on either side of the xyphoid process.
Place a retractor across the abdomen to spread the muscle and skin and expose the entire abdominal area.
Moisten two 7.5 x 7.5 cm2 gauze swabs with sterile physiological saline and spread one across the top of the surgical opening and one across the bottom, as shown in Figure 2A.
4. Isolation of the Hepatic Artery
Using two moistened cotton applicator swabs moistened with sterile physiological saline, gently lift the intestines and cecum out of the abdominal cavity, and place them on the lower gauze swab. Fold the gauze over to keep the organs moist and to hold them out of the way.
Using two moistened cotton applicator swabs, gently lift up the left lateral liver lobe, which will be the most predominant lobe in view at this stage. Flip the lobe upwards, and, using a small pair of spring scissors, cut through the fibrous membrane on the underside of the lobe, allowing the lobe to be completely flipped upwards and rest on top of the previously placed gauze swab. Fold the gauze swab over the lobe to hold it in place.
Continue with the next lobe (the anterior caudate), which is now the most anterior lobe in view. With the aid of a dissecting microscope and fine forceps, dissect the thin membrane surrounding the lobe in order to release it and allow it to be freely flipped upwards. Place the lobe underneath the gauze swab together with the left lateral lobe.
Observe the posterior caudate lobe, which will now be visible. Repeat step 4.3 to completely dissect the posterior caudate lobe and flip it upwards and inside the gauze together with the previously placed liver lobes.
- Just beneath the original position of the caudate lobe, find the common hepatic artery, which will now be visible and easily recognized by its strong pulsation. Following the artery to the anatomical right, observe the thick membranous material obscuring the gastroduodenal and proper hepatic arteries from view. Using fine forceps, lift up this material and gently tear it, taking care to avoid any small vessels in this area. Use cotton applicator swabs to stop any bleeding which might occur.
- Locate the arterial branches, which will be clearly visible with the aid of a dissecting microscope when the membranes are properly dissected.
Using two fine, atraumatic forceps, carefully dissect the common hepatic artery, gastroduodenal artery, and the proper hepatic artery such that they are freed from the surrounding membranes (Figure 2B).
5. Preparation of the Artery for Injection
Pass a 7-0 Prolene suture underneath the gastroduodenal artery, and use a micro-needle-holder to tie a permanent ligature at the distal end, just above the bifurcation (see Figure 1 for proper placement). Allow approximately 2 inches of suture material remaining on the ends, and clamp them with a needle holder to pull the artery taught, as in Figure 2C.
- Place a small clamp on the common hepatic artery to temporarily block blood flow (Figure 2C). Note: This is important to prevent massive bleeding once the needle is removed from the artery. Furthermore, the blockage of arterial blood flow will allow for slower virus administration and better transduction efficiency.
- Important: Work quickly to limit the amount of time that blood flow is stopped - perform the injection and subsequent ligation of the artery within 5 min of clamping the common hepatic artery.
- If possible, increase the magnification on the dissecting microscope, such that the gastroduodenal artery is in sharp focus and appears large enough to accurately insert the 30 G needle. Place the needle of the injection syringe into the lumen of the gastroduodenal artery and slowly push on the plunger. Note: Proper placement of the needle will result in the injected solution flowing directly through the proper hepatic artery and towards the liver.
- Perform the injection very slowly to maximize transduction efficiency.
Once the entire injection volume has been administered, slowly retract the needle from the artery. Note: There should be no bleeding from the injection site. In the case that bleeding does occur, it indicates that the clamp is not properly placed and should be quickly adjusted.
6. Closing
Pass a second 7-0 Prolene suture beneath the gastroduodenal artery, and tie a second permanent ligature above the injection site.
- Remove the clamp from the common hepatic artery. Confirm that blood flow through the proper hepatic artery towards the liver has been restored.
- Cut the loose ends of the ligatures.
Confirm that there is no bleeding before returning the intestines and liver to the abdominal cavity. Take care that the appendix is returned in its original orientation.
- Suture the muscle and skin in separate layers using 4-0 vicryl suture material with a curved needle. Place continuous sutures approximately 1 mm apart and pull tightly.
- During the suturing phase, administer an analgesic such as buprenorphine (0.05 mg/kg).
- Remove rat from anesthesia. If isoflurane has been used, the rat should regain consciousness within several minutes. If MMF has been used as an injectable anesthesia, antagonize it with a subcutaneous injection of Atipamezole (0.75 mg/kg), Flumazenil (0.2 mg/kg), and Naloxone (9.12 mg/kg), which will reverse the effects of the anesthesia and allow the animals to regain consciousness within 20 min. Keep rats warm with an infrared warming lamp during the initial wakening phase.
- Do not leave an animal unattended until it has regained sufficient consciousness to maintain sternal recumbency.
- Do not return an animal that has undergone surgery to the company of other animals until fully recovered.
Monitor rats post-operatively for pathological signs of distress, and follow-up with analgesics as required by the local animal care and use committee. For example, buprenorphine can be administered at 0.05 mg/kg every 12 hr.
Representative Results
With experience, a nearly 100% success rate (meaning that the artery was successfully dissected and injected, and the animal survived surgery) can be achieved. However, the health status of the rat prior to surgery (degree of tumor burden, underlying liver function, etc.) will obviously play a role in the outcome of the surgical intervention. Following surgery, rats can be expected to experience transient weight loss and a slight, transient elevation of liver enzymes, even if buffer alone, without virus, was injected.
We have previously demonstrated that trans-hepatic arterial administration of oncolytic vesicular stomatitis virus (VSV) or Newcastle disease virus (NDV) results in efficient tumor transduction and tumor-specific virus replication, in an orthotopic, multifocal HCC model8,9. Although the two viruses differ in their intratumoral kinetics of virus replication, at the respective time-points of maximal virus replication, numerous foci of virus propagation, as evidenced by positive enzymatic staining for the LacZ reporter gene, could be observed exclusively within HCC nodules, while the surrounding livers showed no evidence of virus replication (Figure 3 and 4, previously published data8,9). Additionally, morphometric analysis of randomly selected VSV-treated tumor nodules demonstrated that tumor transduction efficiency does not correlate with tumor size (Figure 3).
Furthermore, the ability to deliver therapeutic agents through the hepatic artery also offers the possibility to combine therapy with an embolization agent as an alternative to TACE. We have demonstrated that, by mixing oncolytic VSV at a 1:1 ratio with a degradable starch microsphere (DSM) embolization agent and infusing the suspension through the hepatic artery, we could achieve massive tumor necrosis and very significant survival prolongation in rats bearing multifocal HCC nodules11. Trans-hepatic arterial administration of the DSM resulted in a nearly complete embolization of tumor nodules, while having a minimal effect on the perfusion of normal liver tissue, as demonstrated by dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) using a gadolinium contrast agent (Figure 5, previously published data11).
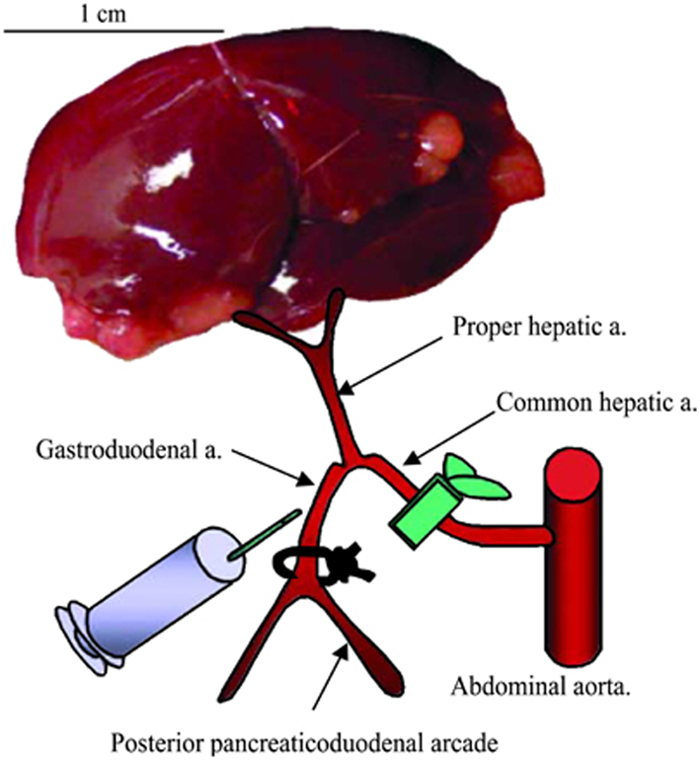 Figure 1. Schematic representation of hepatic arterial infusion procedure. The hepatic vessels (common hepatic artery, proper hepatic artery, and gastroduodenal artery) are dissected with the aid of a dissecting microscope. After ligation of the gastroduodenal artery and temporal block of the common hepatic artery, 1 ml of PBS or VSV vector are administered via the gastroduodenal artery through the proper hepatic artery. After injection, the proximal site of the gastroduodenal artery is ligated to prevent bleeding, the block of the common hepatic artery is released, and the recommencement of hepatic blood flow is confirmed. This figure has been previously published9. Please click here to view a larger version of this figure.
Figure 1. Schematic representation of hepatic arterial infusion procedure. The hepatic vessels (common hepatic artery, proper hepatic artery, and gastroduodenal artery) are dissected with the aid of a dissecting microscope. After ligation of the gastroduodenal artery and temporal block of the common hepatic artery, 1 ml of PBS or VSV vector are administered via the gastroduodenal artery through the proper hepatic artery. After injection, the proximal site of the gastroduodenal artery is ligated to prevent bleeding, the block of the common hepatic artery is released, and the recommencement of hepatic blood flow is confirmed. This figure has been previously published9. Please click here to view a larger version of this figure.
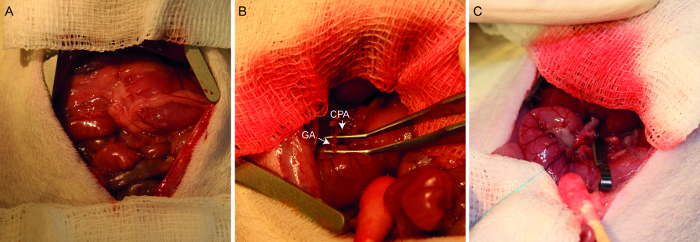 Figure 2. Photographs of the hepatic arterial infusion procedure. A laparotomized rat demonstrates the placement of the abdominal wall retractor and gauze swabs (A). After lifting out the hepatic lobes, the common hepatic artery (designated CHA) and gastroduodenal artery (designated GA) are identified and dissected (B). After ligating the distal end of the gastroduodenal artery, the common hepatic artery is temporarily clamped (C). The gastroduodenal artery is now ready for injection. Please click here to view a larger version of this figure.
Figure 2. Photographs of the hepatic arterial infusion procedure. A laparotomized rat demonstrates the placement of the abdominal wall retractor and gauze swabs (A). After lifting out the hepatic lobes, the common hepatic artery (designated CHA) and gastroduodenal artery (designated GA) are identified and dissected (B). After ligating the distal end of the gastroduodenal artery, the common hepatic artery is temporarily clamped (C). The gastroduodenal artery is now ready for injection. Please click here to view a larger version of this figure.
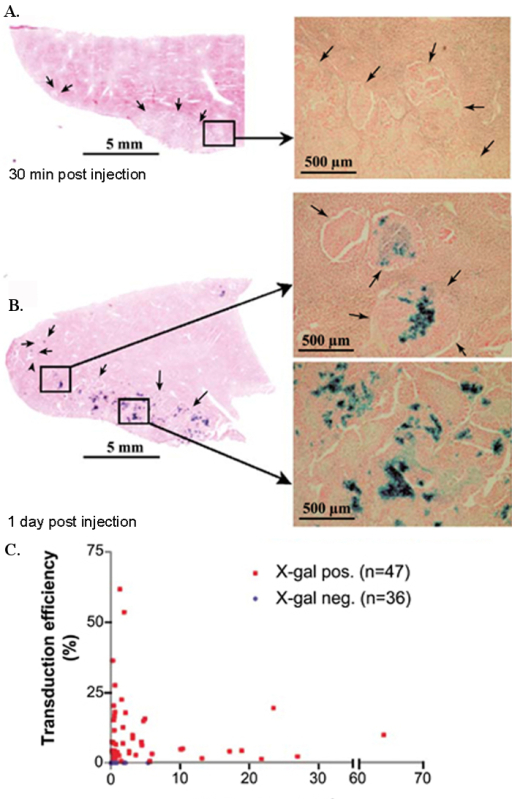 Figure 3. Transduction and tumor-selective replication of VSV after hepatic artery administration in rats bearing multifocal HCC. Sets of animals (n = 3/time point) were sacrificed (A) at 30 min and (B) at day 1 post-virus infusion, and frozen liver sections were stained for β-gal expression. Representative sections are shown at low (left) and higher magnifications (right). Arrows indicate the borders between tumor lesions and hepatic parenchyma. (C) Relationship between tumor size and transduction efficiency. HCC-containing liver sections were analyzed by morphometric analysis to quantify total tumor and X-gal-positive areas. This figure has been previously published9. Please click here to view a larger version of this figure.
Figure 3. Transduction and tumor-selective replication of VSV after hepatic artery administration in rats bearing multifocal HCC. Sets of animals (n = 3/time point) were sacrificed (A) at 30 min and (B) at day 1 post-virus infusion, and frozen liver sections were stained for β-gal expression. Representative sections are shown at low (left) and higher magnifications (right). Arrows indicate the borders between tumor lesions and hepatic parenchyma. (C) Relationship between tumor size and transduction efficiency. HCC-containing liver sections were analyzed by morphometric analysis to quantify total tumor and X-gal-positive areas. This figure has been previously published9. Please click here to view a larger version of this figure.
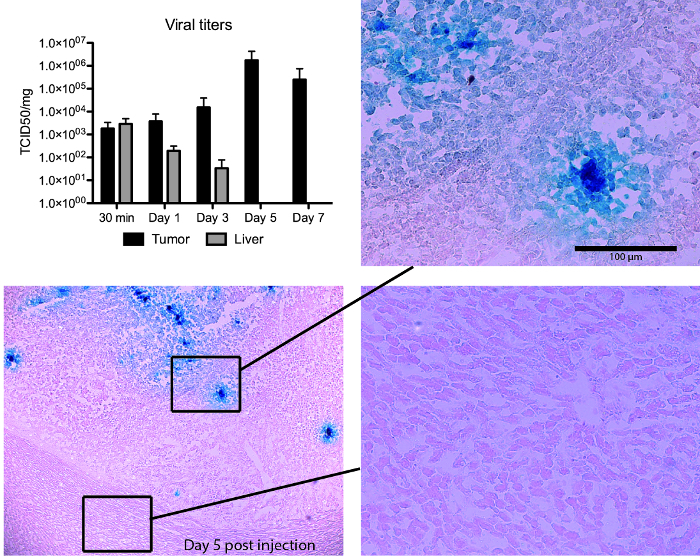 Figure 4. NDV/F3aa(L289A) treatment results in tumor-specific virus replication in hepatocellular carcinoma (HCC)-bearing rats. Male Buffalo rats bearing multifocal orthotopic HCC nodules were treated with rNDV/F3aa(L289A) (108 TCID50) by hepatic arterial infusion, and killed at the indicated time points post-treatment (n = 3). Tumor-containing liver sections were subjected to β-gal staining. Representative sections are shown at 5X (overview) and 20X (magnification of tumor and liver sections) magnification. Viral titers were quantified by TCID50 analysis of liver and tumor lysate, and are expressed as the mean ± SD. NDV, Newcastle disease virus; TCID50, 50% tissue culture infectious dose. Note, this Figure has been modified from its original form8. This figure has been previously published8. Please click here to view a larger version of this figure.
Figure 4. NDV/F3aa(L289A) treatment results in tumor-specific virus replication in hepatocellular carcinoma (HCC)-bearing rats. Male Buffalo rats bearing multifocal orthotopic HCC nodules were treated with rNDV/F3aa(L289A) (108 TCID50) by hepatic arterial infusion, and killed at the indicated time points post-treatment (n = 3). Tumor-containing liver sections were subjected to β-gal staining. Representative sections are shown at 5X (overview) and 20X (magnification of tumor and liver sections) magnification. Viral titers were quantified by TCID50 analysis of liver and tumor lysate, and are expressed as the mean ± SD. NDV, Newcastle disease virus; TCID50, 50% tissue culture infectious dose. Note, this Figure has been modified from its original form8. This figure has been previously published8. Please click here to view a larger version of this figure.
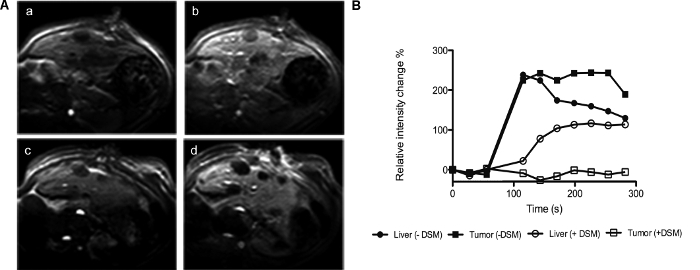 Figure 5. Dynamic contrast-enhanced magnetic resonance imaging of embolized HCC. McA-RH7777 tumor-bearing rats, either nonembolized or 30 min after hepatic arterial embolization with DSM, were imaged with DCE-MRI. (A) Axial T1-weighed precontrast (a, c) and postcontrast (b, d) images show lack of contrast in embolized tumor nodules (d). (B) Data were quantitatively analyzed by measuring gray-scale signal intensities in equal-sized regions of interest of tumor nodules and adjacent normal liver tissue. One representative data set is shown. This figure has been previously published11. Please click here to view a larger version of this figure.
Figure 5. Dynamic contrast-enhanced magnetic resonance imaging of embolized HCC. McA-RH7777 tumor-bearing rats, either nonembolized or 30 min after hepatic arterial embolization with DSM, were imaged with DCE-MRI. (A) Axial T1-weighed precontrast (a, c) and postcontrast (b, d) images show lack of contrast in embolized tumor nodules (d). (B) Data were quantitatively analyzed by measuring gray-scale signal intensities in equal-sized regions of interest of tumor nodules and adjacent normal liver tissue. One representative data set is shown. This figure has been previously published11. Please click here to view a larger version of this figure.
Discussion
Although direct intratumoral injection is undoubtedly the simplest method to result in efficient tumor transduction of a single tumor nodule, hepatic arterial infusion represents an ideal administration route to target multifocal, orthotopic HCC. This method has proven to be both safe and effective for treating HCC in immune competent rats with oncolytic viruses. Furthermore, since HCC patients are routinely treated by transarterial application of chemoembolization, the method described here is readily translatable to a clinical setting.
An obvious disadvantage of hepatic arterial delivery of tumor therapies in preclinical rodent models is the invasiveness of the procedure and the time required, both for the surgical intervention and the post-operative care of the rats. Researchers wishing to establish this technique must invest a substantial amount of time in becoming proficient at the procedure to produce reproducible results and a high survival rate. Furthermore, due to the extremely small diameter of the hepatic artery, to date there have been no reports of hepatic arterial infusions in mice. This is unfortunate, since the genetic models of HCC are primarily generated in mice, and we are therefore limited to testing hepatic arterially applied therapies in implantation or chemically induced HCC models in rats. Chemical induction of HCC is also associated with general liver toxicity, which would potentially preclude the rats from eligibility for receiving this therapeutic intervention due to safety concerns.
As an alternative to the invasive laparotomy approach to hepatic arterial virus delivery, it is theoretically feasible to feed a catheter through the femoral artery and up through the hepatic aorta and into the hepatic artery in rats, which is the standard procedure in patients. Although this approach would provide the huge benefit of avoiding surgical intervention, it is also technically challenging to establish and would require the aid of an experienced interventional radiologist.
Finally, it is noteworthy to mention that the procedure described here, although established for the purpose of efficient delivery of oncolytic viruses to orthotopic HCC, can be applied to any therapeutic agent for locoregional delivery to hepatic lesions, such as chemotherapy, immune-modulatory drugs, or small molecules. This method allows for a sophisticated pre-clinical model for testing novel therapies for clinical translation.
Disclosures
The authors have nothing to disclose.
Acknowledgments
This work is supported by the SFB 824 subprojects C6 and C7 (DFG Sonderforschungsbereich 824), German Research Foundation, Bonn, Germany.
References
- Murray CJ, Lopez AD. Evidence-based health policy - lessons from the Global Burden of Disease Study. Science. 1996;274:740–743. doi: 10.1126/science.274.5288.740. [DOI] [PubMed] [Google Scholar]
- Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94:153–156. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- Bruix J, Sala M, Llovet JM. Chemoembolization for hepatocellular carcinoma. Gastroenterology. 2004;127:179–188. doi: 10.1053/j.gastro.2004.09.032. [DOI] [PubMed] [Google Scholar]
- Miraglia R, Pietrosi G, Maruzzelli L, Petridis I, Caruso S, Marrone G, Mamone G, Vizzini G, Luca A, Gridelli B. Efficacy of transcatheter embolization/chemoembolization (TAE/TACE) for the treatment of single hepatocellular carcinoma. World J Gastroenterol. 2007;13:2952–2955. doi: 10.3748/wjg.v13.i21.2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breedis C, Young G. The blood supply of neoplasms in the liver. Am J Pathol. 1954;30:969–977. [PMC free article] [PubMed] [Google Scholar]
- Nakashima T, Kojiro M. Pathologic characteristics of hepatocellular carcinoma. Semin Liver Dis. 1986;6:259–266. doi: 10.1055/s-2008-1040608. [DOI] [PubMed] [Google Scholar]
- Heo J, et al. Randomized dose-finding clinical trial of oncolytic immunotherapeutic vaccinia JX-594 in liver cancer. Nat Med. 2013;19:329–336. doi: 10.1038/nm.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altomonte J, Marozin S, Schmid RM, Ebert O. Engineered newcastle disease virus as an improved oncolytic agent against hepatocellular carcinoma. Mol Ther. 2010;18:275–284. doi: 10.1038/mt.2009.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki K, Ebert O, Kournioti C, Tai YS, Woo SL. Oncolysis of multifocal hepatocellular carcinoma in the rat liver by hepatic artery infusion of vesicular stomatitis virus. Mol Ther. 2004;9:368–376. doi: 10.1016/j.ymthe.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Shinozaki K, Ebert O, Woo SL. Eradication of advanced hepatocellular carcinoma in rats via repeated hepatic arterial infusions of recombinant VSV. Hepatology. 2005;41:196–203. doi: 10.1002/hep.20536. [DOI] [PubMed] [Google Scholar]
- Altomonte J, et al. Synergistic antitumor effects of transarterial viroembolization for multifocal hepatocellular carcinoma in rats. Hepatology. 2008;48:1864–1873. doi: 10.1002/hep.22546. [DOI] [PubMed] [Google Scholar]


