Abstract
Enzyme-linked immunosorbent assay (ELISA) is a promising method to detect small amount of proteins in biological samples. The devices providing a platform for reduced sample volume and assay time as well as full automation are required for potential use in point-of-care-diagnostics. Recently, we have demonstrated ultrasensitive detection of serum proteins, C-reactive protein (CRP) and cardiac troponin I (cTnI), utilizing a lab-on-a-disc composed of TiO2 nanofibrous (NF) mats. It showed a large dynamic range with femto molar (fM) detection sensitivity, from a small volume of whole blood in 30 min. The device consists of several components for blood separation, metering, mixing, and washing that are automated for improved sensitivity from low sample volumes. Here, in the video demonstration, we show the experimental protocols and know-how for the fabrication of NFs as well as the disc, their integration and the operation in the following order: processes for preparing TiO2 NF mat; transfer-printing of TiO2 NF mat onto the disc; surface modification for immune-reactions, disc assembly and operation; on-disc detection and representative results for immunoassay. Use of this device enables multiplexed analysis with minimal consumption of samples and reagents. Given the advantages, the device should find use in a wide variety of applications, and prove beneficial in facilitating the analysis of low abundant proteins.
Keywords: Bioengineering, Issue 110, lab-on-a-disc, electrospinning, TiO2 nanofibers, immunoassay, full automation, transfer-printing, on-disc detection, low-volume assay, whole blood, ultrasensitive
Introduction
Several platforms for disease diagnosis have been developed based on nanoscale materials1,2 such as nanowires,3 nanoparticles,4 nanotubes,5 and nanofibers (NFs)6-8. These nanomaterials offer excellent prospects in the design of new technologies for highly sensitive bioassays owing to their unique physicochemical properties. For example, mesoporous zinc oxide nanofibers have been used for the femto-molar sensitive detection of breast cancer biomarkers.9 Recently, nanomaterials based on titanium dioxide (TiO2) have been explored for bioanalytical applications10 considering their chemical stability,11 negligible protein denaturation,12 and biocompatibility.13 In addition, the hydroxyl groups on the surface of TiO2 facilitate chemical modification and the covalent attachment of biomolecules.14,15 Patterned TiO2 thin films16 or TiO2 nanotubes17 have been utilized to enhance the detection sensitivity of a target protein by increasing the surface area; however, the fabrication process is rather complex and requires expensive equipment. On the other hand, electrospun NFs are receiving attention because of their high surface area as well as straightforward and low-cost fabrication process;18,19 yet, the fragile or loose characteristic of the electrospun TiO2 NF mat makes it difficult to handle and integrate with microfluidic devices.6,20 Therefore, the TiO2 NF mats were rarely utilized in bioanalytical applications, particularly those requiring harsh washing conditions.
In this study, to overcome these limitations, we have developed a new technology for transferring the electrospun NF mats onto the surface of any target substrate by utilizing a thin polydimethylsiloxane (PDMS) adhesive layer. Furthermore, we have successfully showed the integration of electrospun TiO2 NF mats onto a centrifugal microfluidic device made of polycarbonate (PC). Using this device, a high-sensitive, fully automated, and integrated detection of C-reactive protein (CRP) as well as cardiac troponin I (cTnI) was achieved within 30 min from only 10 μL of whole blood.21 Due to the combined advantages of the properties of the NFs and the centrifugal platform, the assay exhibited a wide dynamic range of six orders of magnitude from 1 pg/ml (~8 fM) to 100 ng/ml (~0.8 pM) with a lower limit of detection of 0.8 pg/ml (~6 fM) for CRP and a dynamic range from 10 pg/ml (~0.4 pM) to 100 ng/ml (~4 nM) with a detection limit of 37 pg/ml (~1.5 pM) for cTnI. These detection limits are ~300 and ~20-fold lower compared to their corresponding conventional ELISA results. This technique could be applied for the detection of any target proteins, with appropriate antibodies. Overall, this device could contribute greatly to in-vitro diagnostics and biochemical assays since it can detect rare amounts of target proteins with great sensitivity even from very small quantities of biological samples; e.g., 10 μl of whole blood. Though we only demonstrated the serum protein detection using ELISA in this study, the transfer and integration technology of electrospun NFs with microfluidic devices could be more broadly applied in other biochemical reactions which require a large surface area for high detection sensitivity.
Protocol
NOTE: Blood was drawn from healthy individuals and was collected in a blood collection tube. Written informed consent was obtained from all volunteers.
1. Fabrication of TiO2 NF Mat
- Preparation of precursor solution22
- Dissolve 1.5 g of titanium tetraisopropoxide (TTIP) in a mixture of ethanol (99.9%, 3 ml) and glacial acetic acid (3 ml) and mix the solution at RT (25 °C) for 30 min on a magnetic stirrer.
- Dissolve 11 wt% of polyvinyl pyrrolidone (PVP) in 3.64 g of ethanol (99.9%) and stir the solution at 65 °C for 1 hr using a hot-plate magnetic stirrer.
- Combine and stir the two solutions at 65 °C for 4 hr on a hot-plate magnetic stirrer.
- Electrospinning
- Load the prepared solution into a syringe connected to a stainless steel needle of 200 μm inner diameter. Pour the solution directly into the syringe after removing the plunger.
- Introduce the solution constantly at a flow rate of 0.3 ml/hr for 10 min onto a Si wafer (2 cm x 2 cm) placed on a grounded substrate at a high DC voltage (15 kV). During the electrospinning, set the distance between the needle and the grounded substrate to 10 cm.
- Calcination of the NF mat
- Place the NF mat in a furnace and increase the temperature up to 500 °C with a ramping rate of 3 °C/min under high-vacuum condition (5 x 10-5 torr).
- Maintain the temperature at 500 °C for 3 hr. Cool down the temperature of the samples to the RT (25 °C) in the furnace.
2. Integration of TiO2 NF Mat into a Centrifugal Microfluidic Disc
- Fabrication of a disc
- Use the 3D designing software (e.g., SolidWorks or similar) to depict chambers, channels, or valves of each layer of disc. Convert the design files to computer numerical control (CNC) code using code generating software (e.g., Edgecam or similar). Use the softwares according to the instruction manual.23,24 Note: The typical channel dimensions used in this device are 1.0 mm x 0.3 mm (width x depth).
- Open the operating software of CNC milling machine (e.g.,DeskCNC or similar) and load CNC code to the operating software and click the following in order: "AUTO" -> "G CODE OPEN" -> Select the generated CNC code.
- Attach the PC plate on the CNC milling machine: use 1 mm PC plates for the top layer and 5 mm PC plates for the disc layer. Run the CNC milling machine to cut each layer of the disc by clicking the "START" button.
- Transfer of TiO2 NF mat onto the disc
- Place a silicon substrate and a small petri dish containing 40 µL of (tridecafluoro-1,1,2,2-tetrahydrooctyl)-1-trichlorosilane in a vacuum chamber under vacuum for 30 min. The fluorosilane vaporizes and gets coated on the substrate.
- Mix polydimethylsiloxane (PDMS) pre-polymer with a curing agent in a 10:1 ratio and degas in a vacuum chamber. Spin-coat the PDMS onto a silicon substrate at 650 rpm for 60 sec.
- Cure the PDMS coated on silicon substrate in an oven at 65 °C for 10 min to achieve an adherent state. NOTE: Curing time can be varied depending on environmental conditions, and adhesion force can be tested by a tack test using a rheometer.
- Transfer the TiO2 NF mat prepared by electrospinning and calcination onto the PDMS-coated silicon using a tweezer. Then, manually apply a conformal pressure on the transferred TiO2 NF mat with a flat object (e.g., glass slide) and remove the object. Put this sample in the oven at 65 °C for 4 hr or at 80 °C for 1 hr to cure the PDMS layer completely.
- Coat binding reaction chambers in the disc with 20 µl the PDMS/curing agent mixture (10:1), which acts as an adhesive layer to attach TiO2 NF mat onto the disc.
- Dispense 50 µl of ethanol (99.9%) on the TiO2 NF mat on the PDMS layer, cut the NF mat with a punch hole of 6 mm diameter, and transfer the cut TiO2 NF mat to the binding reaction chambers coated with 20 µl of the PDMS in the previous step. NOTE: In this step, ethanol will be helpful to detach the cut TiO2 NF mat from the Si substrate.
- Incubate the TiO2 NF mat integrated disc in the oven at 65 °C for 4 hr or at 80 °C for 1 hr to cure the PDMS completely.
- Surface modification of the TiO2 NF mat on the disc
- Prepare 1% v/v solution of (3-glycidoxypropyl)methyldiethoxy silane (GPDES) in ethanol (99.9%).
- Treat the TiO2 NF mat integrated disc with oxygen plasma at 140 W, 50 sccm oxygen flow for 180 sec. Dispense 100 μl of GPDES solution on each nanofiber mat and incubate at RT (25 °C) for 2 hr.
- Wash the substrates briefly by dispensing ethanol (99.9%) using a wash bottle, then remove the ethanol completely by inverting the disc and blotting it against a clean wipe. Cure at 80 °C for 1 hr. Wash twice with ethanol (99.9%) in the same manner as stated above, to remove the physically adsorbed and unbound GPDES molecules.
- Blow dry with a nitrogen stream, dry under vacuum. NOTE: The disc can be stored in a sealed container at RT (25 °C) until use.
- Immobilization of antibodies on the surface
- Make a solution of 200 μg/ml of capture antibodies (monoclonal mouse antihuman hsCRP or monoclonal mouse anti-cTnI) by diluting the antibodies with a phosphate buffered saline (PBS) buffer (pH 7.4). Dispense 5 μl of the solution onto each NF mat in a disc using a micropipette. See materials list for more information about the antibodies.
- Keep the discs in a humidified chamber and incubate at 37 °C for 4 hr. Wash the antibodies coated NF mat with 0.1% BSA-PBS buffer. Fill the chamber with 100 μl of wash buffer using a micropipette, remove the buffer by aspirating or decanting. Finally, invert the disc and blot it against a clean wipe, and then assemble the disc.
- Disc assembly
- Draw the design of the disc on double-side adhesive tape using a CAD program (e.g., AutoCAD or similar). Load the CAD design to the cutting plotter.
- Cut the double-side adhesive tape using the cutting plotter. Peel off one protection layer of double adhesive tape and attach it on top of the disc layer. Peel off the other protection layer and attach the top layer on the disc layer.
- Load the preliminarily assembled disc in the pressing machine and precisely align top/adhesive/disc layers using align marks in each layer to connect each valve, channel, and chambers. Apply conformal pressure using the pressing machine.
3. Immunoassay
Fill the chambers with 1% BSA-PBS buffer using a micropipette and incubate the disc at 37 °C for 1 hr. Remove the buffer by aspiration using a micropipette. Perform this step to block the un-reacted sites and to reduce non-specific adsorption of protein in a disc.
Wash twice with 0.1% BSA-PBS by filling and aspirating the chambers using a micropipette. NOTE: At this stage the disc can be stored at 4 °C until use.
Load 10 μl of antigen-spiked whole blood or CRP-free serum for CRP detection on the disc using a micropipette. For making the calibration graphs, use concentrations of CRP from 1 pg/ml to 100 ng/ml; and cTnI from 10 pg/ml to 100 ng/ml. NOTE: Due to the higher levels of CRP in whole blood, which is usually in µM range, CRP-free serum was used to demonstrate the low detection limit of the device.
Spin the disc at 3,600 rpm (391 x g) for 60 sec to separate the red blood cells.
Open valve #1 by laser irradiation, and transfer 4 μl of the supernatant plasma to the chamber containing 8 μl of detecting antibodies conjugated with HRP by spinning the disc at 2,400 rpm for 3 sec. NOTE: The general processes for valve actuation and visualization of disc operation are described in detail in a previous report.21
Apply a mixing mode (15 Hz s-1, 15°) for 5 sec for binding of the protein and detection antibodies.
Open valve #2, and transfer the mixture prepared in step 6 to the binding reaction chamber (2,400 rpm, 3 sec). Then, apply a mixing mode (60 Hz s-1, 2°) for 20 min to achieve an immunoreaction between the mixture and the binding antibodies on the TiO2 NFs. After the reaction, open valve #3 and transfer the residual mixture to the waste chamber (2,400 rpm, 10 sec).
Transfer the washing buffer (600 µl) to the binding reaction chamber by opening valve #4 and spinning the disc (2,400 rpm, 4 sec). Then apply a mixing mode (30 Hz s-1, 30°) for 120 sec to wash the TiO2 NFs in the chamber.
Wash the TiO2 NFs twice more by following same condition of step 3.8, and remove the residual washing buffer completely by spinning the disc at 2,400 rpm for 20 sec. Then, close the valve #5.
For the chemiluminescent reaction, apply a mixing mode (30 Hz s-1, 2°) for 1 min after transferring the chemiluminescent substrate solution to the binding reaction chamber by opening valve #6 and spinning the disc at 2,400 rpm for 10 sec subsequently.
Transfer the reacted substrate solution to the detection chambers by opening valve #7 and spinning the disc at 2,400 rpm for 10 sec.
Measure chemiluminescence signals from the reacted substrate solution using a photomultiplier tube (PMT) module equipped detection system. NOTE: The detection system is a custom-built machine using the following parts: step motor, optomechanical components, translation stages, and commercially available PMT. The optomechanical parts and step motor are assembled to hold the device, and the motor can rotate the disc. The detection is performed by PMT fixed on the optomechanical parts, and it is located above the detection chambers of the device. By manipulating the step motor, the detection chambers are aligned right below the detection zone of PMT.
Representative Results
Using this protocol, a fully automated centrifugal microfluidic device for protein detection from whole blood with high sensitivity was prepared. The TiO2 NF mats were prepared by processes of electrospinning and calcination. In order to fabricate the NFs of desired diameter, morphology, and thickness, electrospinning conditions such as flow rate, voltage, and spinning time were optimized. When the conditions were not optimized, the quality of the NFs formed was poor. In particular, NFs were not spun out from the polymer solution at low voltage (5 kV), and were broken when the voltage was high (>20 kV). Similarly, spinning time was critical for the fabrication of NFs with appropriate density, not too sparse (1 min) or too dense (30 min). Also, to avoid bead formation caused by high flow rates, the flow rate was manipulated. So, an applied voltage of 15 kV, 10 min electrospinning time and a flow rate of 0.3 ml hr-1 were used as optimal conditions for NF fabrication based on the morphology and thickness of the resulting NFs (Figure 1).
The TiO2 NF mat was successfully transferred onto the target substrate using an adhesive PDMS layer, which was prepared by spin-coating PDMS on a silanized silicon substrate and pre-curing it in an oven at 65 °C. The time for pre-curing the PDMS layer was optimized by checking the adhesion force, using a tack test (Figure 2A). Figure 2B shows the effect of curing time on nanofiber attachment. If the PDMS was heated for 3 min, there is no adhesion force as the PDMS was still uncured and remains as a liquid and the NFs got embedded into the PDMS layer. When it is heated for 30 min, the adhesion force is very weak as the PDMS becomes stiff and loses its sticky property and the NFs were not attached on it. When the PDMS was heated for 10 min, the PDMS showed strong adhesion force and the NFs were attached strongly. From the results, it is concluded that the optimal time for pre-curing PDMS for strong attachment is 10 min.
After disc assembly, the immunoassay was conducted on the TiO2 NF mat integrated disc by following a series of disc operation processes as listed in Table 1. For the determination of CRP and cTnI concentrations in unknown samples, calibration graphs for each were made by plotting the relative light units (RLU) versus the CRP or cTnI concentration (Figure 3). In Figure 3A and 3B, the on-disc assay results were compared with conventional ELISA on a 96-well plate for CRP and cTnI respectively. The assays exhibited a broad linear dynamic range with a detection limit of 0.8 pg/ml (~6 fM) for CRP and 37 pg/ml (1.5 pM) for cTnI on a disc, which is about 300 times higher for CRP and 20 times higher for cTnI compared to their respective conventional ELISA results (286 pg/ml, 2.3 pM for CRP; 824 pg/ml, 32 pM for cTnI).
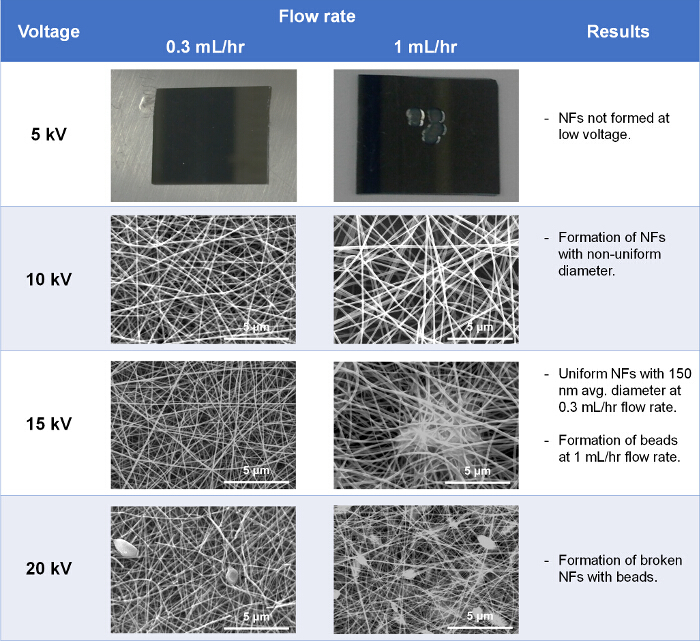
Figure 1. Optimization of electrospinning conditions. SEM images at each condition show the morphology of NFs formed. Please click here to view a larger version of this figure.
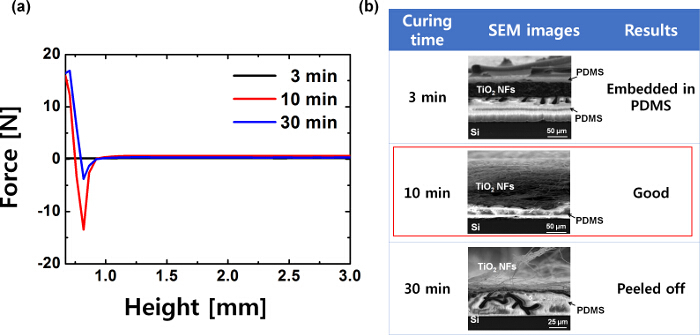 Figure 2. Tack test of adhesive PDMS layer and SEM images of the transferred TiO2 NF mat. (A) Adhesion force of the adhesive PDMS layer, pre-cured for several lengths of time, (B) SEM images of nanofibers attached on PDMS cured for different lengths of time. This figure has been modified from Ref. 21. Please click here to view a larger version of this figure.
Figure 2. Tack test of adhesive PDMS layer and SEM images of the transferred TiO2 NF mat. (A) Adhesion force of the adhesive PDMS layer, pre-cured for several lengths of time, (B) SEM images of nanofibers attached on PDMS cured for different lengths of time. This figure has been modified from Ref. 21. Please click here to view a larger version of this figure.
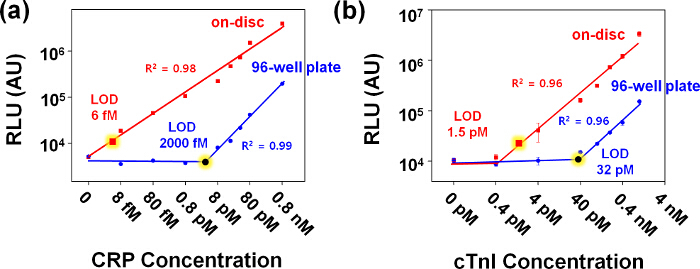 Figure 3. Calibration graphs for the detection of CRP and cTnI using lab-on-a-disc and 96-well plate. Detection of CRP spiked in CRP-free serum (A) and cTnI spiked in whole blood (B) were conducted. The error bars indicate the standard deviation of at least three independent measurements. The limit of detection (LOD) was calculated by 3 times of the standard deviation of the negative control data measured with CRP-free serum or whole blood without cTnI spiking for CRP and cTnI, respectively. Please click here to view a larger version of this figure.
Figure 3. Calibration graphs for the detection of CRP and cTnI using lab-on-a-disc and 96-well plate. Detection of CRP spiked in CRP-free serum (A) and cTnI spiked in whole blood (B) were conducted. The error bars indicate the standard deviation of at least three independent measurements. The limit of detection (LOD) was calculated by 3 times of the standard deviation of the negative control data measured with CRP-free serum or whole blood without cTnI spiking for CRP and cTnI, respectively. Please click here to view a larger version of this figure.
| Spin No. | Speed (rpm) | Valve No. | Time (sec) | Operation |
| 1 | 3,600 | 60 | blood separation | |
| 2 | 2,400 | 1 | 3 | open valve to transfer the plasma into the chamber containing 8 µl of detecting antibodies conjugated with HRP |
| 3 | 15 Hz, 15° | 5 | mix plasma and detecting antibodies | |
| 4 | 2,400 | 2 | 3 | open valve to transfer the mixture into binding reaction chamber |
| 5 | 60 Hz, 2° | 1,200 | mix capture antibodies on TiO2 NF mat and plasma-detecting antibodies mixture | |
| 6 | 2,400 | 3 | 10 | open valve to remove the mixture |
| 7 | 2,400 | 4 | 4 | open valve to transfer washing buffer |
| 8 | 30 Hz, 30° | 120 | mix washing buffer and wash TiO2 NF mat | |
| 9 | 2,400 | 4 | transfer washing buffer | |
| 10 | 30 Hz, 30° | 120 | mix washing buffer and wash TiO2 NF mat | |
| 11 | 2,400 | 4 | transfer washing buffer | |
| 12 | 30 Hz, 30° | 120 | mix washing buffer and wash TiO2 NF mat | |
| 13 | 2,400 | 4 | transfer washing buffer | |
| 14 | 30 Hz, 30° | 120 | mix washing buffer and wash TiO2 NF mat | |
| 15 | 2,400 | 20 | remove the remaining washing buffer | |
| 16 | 5 | close valve | ||
| 17 | 2,400 | 6 | 10 | open valve and transfer the chemiluminescent substrate |
| 18 | 30 Hz, 2° | 60 | mix substrate and the immunoreagents on TiO2 NF mat | |
| 19 | 2,400 | 7 | 10 | open valve and transfer the reacted substrate to the detection chamber |
| Total time | ~ 30 min |
Table 1. Operation program for the immunoassay in a disc. This table has been modified from Ref. 21.
Discussion
The assay on TiO2 NF integrated disc is a rapid, inexpensive and convenient technique for the ultrasensitive detection of low abundant proteins present in very low volume of blood. This technique has the advantage of using small sample volumes (10 μl) and is amenable for analysis of multiple samples simultaneously. This provides a great potential as a multiplexing immunoassay device. The device has the added advantage that it does not require sample pretreatment steps like plasma separation, which are required in conventional ELISAs. Moreover, the device can perform whole immunoassay procedures (e.g., mixing, washing, binding etc.) automatically due to pre-designed chambers, channels, and valves. Additionally, using commercialized disc fabrication methods (e.g., injection molding, UV/ultrasonic/thermal bonding) instead of milling and manual assembly using adhesives, the whole procedure from device fabrication to analysis can be automated.
Also, the transfer-printing method presented here shows a simple and easy transfer of electrospun NF from donor to target substrate by utilizing a thin adhesive PDMS layer. In general, the TiO2 NFs obtained after the calcination process are brittle and easily peel off from the grounded substrate due to weak adhesion.25 Due to its characteristic brittleness, integrating the TiO2 NF mats with other devices is difficult. To solve this, many methods have been reported. For example, hot-press26 and solvent-vapor27 techniques showed the enhancement of the adhesion between TiO2 NFs mat and target surface before calcination process. Even though these methods increased the adhesion, the TiO2 NF mats were not intact due to the high mechanical pressure or the solvent applied during the respective techniques. Furthermore, because of the calcination step, integration of the TiO2 NF mats using these methods can be applied only for limited target surfaces.
To the best of our knowledge, this is the first technique to show the transfer-printing of NF mats on to a device made of thermoplastics, retaining the novel properties of NFs even after the transfer. For better performance of the device, fabrication of high-quality NFs and optimization of pre-curing conditions are desired. As mentioned in the protocol, the time required for pre-curing may vary depending on the environmental conditions; therefore, pre-curing conditions are to be optimized by measuring the adhesion force using a tack-test.
Furthermore, the protocol presented here, provides skillful guides for the fully automated ELISA process on a disc. The protocol introduces not only fabrication of the disc but also the automation of all the processes required for ELISA, including separation of whole blood, metering, mixing, washing and detection. Each step is optimized with spin speed, mixing frequency, and operation time. Based on this operation guide, the reagents loaded on a disc can be transferred utilizing a single motor. Since the device performed excellent detection sensitivity with very low volume of blood, it provides a great potential for diagnostic applications by screening biomarkers at an early stage of disease. The device would be enabled to detect any biomarker depending on the availability of its specific antibodies.
Although the device with TiO2 NF mats achieved highly enhanced sensitivity due to the large surface area of the mats, one of the remaining challenge of this technique could be in the mass production of uniform NF mats. Because the sensitivity of the ELISA is strongly affected by the surface area of the nanofiber mat, it is critical to achieve not only a high surface area but also a reproducible and uniform surface area in different batches of mass production.
In conclusion, we have shown a simple method to integrate a brittle NF mat into a functional device for ultrasensitive protein detection. The advantages of this method are as follows: 1) previously electrospun TiO2 NFs could be prepared only on conductive and thermally stable surfaces; here, we have shown that they can be transferred onto any substrate including non-conductive and plastic materials; 2) the TiO2 NF mats can withstand pressure and remain stable during washing steps due to the high adhesion force of PDMS layer; 3) it provides a relatively higher surface area for bioassays; and 4) the antibodies can be covalently attached to the TiO2 NFs due to the intact surface properties on NFs. This technique has great potential to be used for integration of nanofibrous materials into devices for diverse applications.
Disclosures
The authors have nothing to disclose.
Acknowledgments
This work was supported by National Research Foundation of Korea (NRF) grants (2013R1A2A2A05004314, 2012R1A1A2043747), a grant from the Korean Health Technology R&D Project, Ministry of Health & Welfare (A121994) and IBS-R020-D1 funded by the Korean Government.
References
- Zhang Y, et al. Nanomaterials for Ultrasensitive Protein Detection. Adv. Mater. 2013;25(28):3802–3819. doi: 10.1002/adma.201301334. [DOI] [PubMed] [Google Scholar]
- Hu W, Li CM. Nanomaterial-based advanced immunoassays. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2011;3(2):119–133. doi: 10.1002/wnan.124. [DOI] [PubMed] [Google Scholar]
- Yang-Kyu C, Chang-Hoon K. Biosensors and Cancer. Science Publishers; 2012. Silicon Nanowire Biosensor for Cancer Markers; pp. 164–183. [Google Scholar]
- Baltazar R, Vistas CR, Ferreira GM. Nanocomposite Particles for Bio-Applications. Pan Stanford Publishing; 2011. Biosensing Applications Using Nanoparticles; pp. 265–282. [Google Scholar]
- Roy P, Berger S, Schmuki P. TiO2 Nanotubes: Synthesis and Applications. Angew. Chem. Int. Ed. 2011;50(13):2904–2939. doi: 10.1002/anie.201001374. [DOI] [PubMed] [Google Scholar]
- Yang D, et al. Electrospun Nanofibrous Membranes: A Novel Solid Substrate for Microfluidic Immunoassays for HIV. Adv. Mater. 2008;20(24):4770–4775. [Google Scholar]
- Chantasirichot S, Ishihara K. Electrospun phospholipid polymer substrate for enhanced performance in immunoassay system. Biosens. Bioelectron. 2012;38(1):209–214. doi: 10.1016/j.bios.2012.05.029. [DOI] [PubMed] [Google Scholar]
- Zhang N, et al. Electrospun TiO2 Nanofiber-Based Cell Capture Assay for Detecting Circulating Tumor Cells from Colorectal and Gastric Cancer Patients. Adv. Mater. 2012;24(20):2756–2760. doi: 10.1002/adma.201200155. [DOI] [PubMed] [Google Scholar]
- Ali MA, Mondal K, Singh C, Dhar Malhotra B, Sharma A. Anti-epidermal growth factor receptor conjugated mesoporous zinc oxide nanofibers for breast cancer diagnostics. Nanoscale. 2015;7(16):7234–7245. doi: 10.1039/c5nr00194c. [DOI] [PubMed] [Google Scholar]
- Mondal K, Ali MA, Agrawal VV, Malhotra BD, Sharma A. Highly Sensitive Biofunctionalized Mesoporous Electrospun TiO2 Nanofiber Based Interface for Biosensing. ACS Appl. Mater. Interfaces. 2014;6(4):2516–2527. doi: 10.1021/am404931f. [DOI] [PubMed] [Google Scholar]
- Tu W, Dong Y, Lei J, Ju H. Low-Potential Photoelectrochemical Biosensing Using Porphyrin-Functionalized TiO2 Nanoparticles. Anal. Chem. 2010;82(20):8711–8716. doi: 10.1021/ac102070f. [DOI] [PubMed] [Google Scholar]
- Liu S, Chen A. Coadsorption of Horseradish Peroxidase with Thionine on TiO2 Nanotubes for Biosensing. Langmuir. 2005;21(18):8409–8413. doi: 10.1021/la050875x. [DOI] [PubMed] [Google Scholar]
- Portan DV, Kroustalli AA, Deligianni DD, Papanicolaou GC. On the biocompatibility between TiO2 nanotubes layer and human osteoblasts. J.Biomed.Mater.Res. Part A. 2012;100(10):2546–2553. doi: 10.1002/jbm.a.34188. [DOI] [PubMed] [Google Scholar]
- Dettin M, et al. Covalent surface modification of titanium oxide with different adhesive peptides: Surface characterization and osteoblast-like cell adhesion. J. Biomed. Mater. Res. Part A. 2009;90(1):35–45. doi: 10.1002/jbm.a.32064. [DOI] [PubMed] [Google Scholar]
- Kim W-J, et al. Enhanced Protein Immobilization Efficiency on a TiO2 Surface Modified with a Hydroxyl Functional Group. Langmuir. 2009;25(19):11692–11697. doi: 10.1021/la901615e. [DOI] [PubMed] [Google Scholar]
- Son KJ, Ahn SH, Kim JH, Koh W-G. Graft Copolymer-Templated Mesoporous TiO2 Films Micropatterned with Poly(ethylene glycol) Hydrogel: Novel Platform for Highly Sensitive Protein Microarrays. ACS Appl. Mater. Interfaces. 2011;3(2):573–581. doi: 10.1021/am101141z. [DOI] [PubMed] [Google Scholar]
- Kar P, Pandey A, Greer JJ, Shankar K. Ultrahigh sensitivity assays for human cardiac troponin I using TiO2 nanotube arrays. Lab Chip. 2012;12(4):821–828. doi: 10.1039/c2lc20892j. [DOI] [PubMed] [Google Scholar]
- Agarwal S, Wendorff JH, Greiner A. Use of electrospinning technique for biomedical applications. Polymer. 2008;49(26):5603–5621. [Google Scholar]
- Ding B, Wang M, Wang X, Yu J, Sun G. Electrospun nanomaterials for ultrasensitive sensors. Mater. Today. 2010;13(11):16–27. doi: 10.1016/S1369-7021(10)70200-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Yang D, Yu T, Jiang X. Incorporation of electrospun nanofibrous PVDF membranes into a microfluidic chip assembled by PDMS and scotch tape for immunoassays. ELECTROPHORESIS. 2009;30(18):3269–3275. doi: 10.1002/elps.200900128. [DOI] [PubMed] [Google Scholar]
- Lee WS, Sunkara V, Han J-R, Park Y-S, Cho Y-K. Electrospun TiO2 nanofiber integrated lab-on-a-disc for ultrasensitive protein detection from whole blood. Lab Chip. 2015;15(2):478–485. doi: 10.1039/c4lc00900b. [DOI] [PubMed] [Google Scholar]
- Li D, Xia Y. Fabrication of Titania Nanofibers by Electrospinning. Nano Lett. 2003;3(4):555–560. [Google Scholar]
- Lombard M. SolidWorks 2013 BIBLE. Indianapolis, IN: John Wiley & Sons Inc; 2013. [Google Scholar]
- Tickoo S. EdgeCAM 11.0 for Manufacturers. Schererville, IN: CADCIM Technologies; 2007. [Google Scholar]
- Zhu R, et al. Improved adhesion of interconnected TiO2 nanofiber network on conductive substrate and its application in polymer photovoltaic devices. Appl. Phys. Lett. 2008;93(1):013102. [Google Scholar]
- Song MY, Ahn YR, Jo SM, Kim DY, Ahn J-P. TiO2 single-crystalline nanorod electrode for quasi-solid-state dye-sensitized solar cells. Appl. Phys. Lett. 2005;87(11):113113. [Google Scholar]
- Katsuhiro O, et al. Electrospinning processed nanofibrous TiO2 membranes for photovoltaic applications. Nanotechnology. 2006;17(4):1026–1031. doi: 10.1088/0957-4484/17/4/030. [DOI] [PubMed] [Google Scholar]


