Abstract
Like various stressors, the addictive use of nicotine (NC) is associated with emotional symptoms such as anxiety and depression, although the underlying mechanisms have not yet been fully elucidated due to the complicated involvement of target neurotransmitter systems. In the elicitation of these emotional symptoms, the fundamental involvement of epigenetic mechanisms such as histone acetylation has recently been suggested. Furthermore, among the interacting neurotransmitter systems implicated in the effects of NC and stressors, the endocannabinoid (ECB) system is considered to contribute indispensably to anxiety and depression. In the present study, the epigenetic involvement of histone acetylation induced by histone deacetylase (HDAC) inhibitors was investigated in anxiety- and depression-related behavioral alterations caused by NC and/or immobilization stress (IM). Moreover, based on the contributing roles of the ECB system, the interacting influence of ECB ligands on the effects of HDAC inhibitors was evaluated in order to examine epigenetic therapeutic interventions. Anxiety-like (elevated plus-maze test) and depression-like (forced swimming test) behaviors, which were observed in mice treated with repeated (4 days) NC (subcutaneous 0.8 mg/kg) and/or IM (10 min), were blocked by the HDAC inhibitors sodium butyrate (SB) and valproic acid (VA). The cannabinoid type 1 (CB1) agonist ACPA (arachidonylcyclopropylamide; AC) also antagonized these behaviors. Conversely, the CB1 antagonist SR 141716A (SR), which counteracted the effects of AC, attenuated the anxiolytic-like effects of the HDAC inhibitors commonly in the NC and/or IM groups. SR also attenuated the antidepressant-like effects of the HDAC inhibitors, most notably in the IM group. From these results, the combined involvement of histone acetylation and ECB system was shown in anxiety- and depression-related behaviors. In the NC treatment groups, the limited influence of SR against the HDAC inhibitor-induced antidepressant-like effects may reflect the characteristic involvement of histone acetylation within the NC-related neurotransmitter systems other than the ECB system.
Introduction
Tobacco use has been the leading global cause of preventable death due to a number of chronic diseases (e.g. cancer and lung/cardiovascular diseases), and is associated with lethality in approximately 6 million people every year [1, 2]. The addictive use of tobacco is sustained due to nicotine (NC), a highly addictive psychoactive ingredient [1], and the chronic use of NC has been reported to result in increased emotional symptoms such as anxiety and depression [3, 4]. Anxiety and depression are representatively observed as withdrawal symptoms in dependent smokers [5–7]. Furthermore, in some daily smokers, direct anxiogenic and depressogenic effects, which disappear following smoking cessation, have been reported [8–10], and the involvement of the combined activation and desensitization of nicotinic acetylcholine receptors (nAChRs) was suggested in the direct causal link between smoking and emotional symptoms using several rodent experimental models [11, 12]. On the other hand, NC-induced anxiolytic and antidepressant effects have also been reported depending on the experimental model, the route of NC administration and the time course of administration [3, 13–17], and these effects are thought to characteristically reinforce the habitual use of NC.
Anxiety and depression are also observed as frequent psychiatric outcomes of various stressors in humans and associated with inappropriate regulation of brain stress systems [18, 19]. In addictive smokers, the dysregulated stress response in the brain similar to cases exposed to stressors has been reported and stressor-like effects of NC were demonstrated [3, 4, 20]. Furthermore, in several epidemiological and experimental studies, exacerbation of emotional symptoms such as anxiety and depression has been reported in certain stressor-exposed smokers [21–23]. However, depending on the type of NC and/or stressor treatment, stress-related anxiety and depression were decreased by cigarette smoking [24]. Also, in some rodent models, anxiety- and depression-like behaviors caused by stressors were antagonized by NC [25, 26]. With respect to these paradoxical interactions between NC and stressors, complicated mechanisms underlying the effects of NC, which are associated with a characteristically altered combination of nAChR activation plus desensitization and subsequent modulation of the stress-related neurotransmitter/neuroendocrine systems [3, 4], seemed to be involved, but the details of the relevant mechanisms have not been elucidated. Nevertheless, the data from behavioral studies on the interactions between the stress-related effects of NC and other stressors seem to contribute, at least in part, to understanding the involved mechanisms, predicting the risk of exacerbated NC effects in stressor-exposed smokers, and improving the ability to treat the NC addiction.
“Epigenetics” was originally defined in 1942 as studies on the developmental processes between genotypes and phenotypes [27], and is currently regarded as studies on the reversible regulation of gene expression that occurs throughout the lifecycle of an organism independently of the DNA sequence [28–30]. Epigenetic mechanisms include processes such as DNA methylation, histone modifications (acetylation, methylation, phosphorylation etc.), and alterations in microRNAs (small, non-coding RNAs) [29–32]. Although the epigenetic involvement in the addiction-related effects of NC has not been sufficiently explored, an increasing number of studies suggest a pivotal contribution of epigenetic modifications such as histone acetylation in the brain to the behavioral alterations induced by NC (i.e. conditioned place preference and self-administration) [33, 34]. Furthermore, as recently reviewed, growing evidence suggests that stress-related anxiety and depression are robustly associated with altered epigenetic processes [35, 36].
Among the neurotransmitter systems involved in the effects of NC, in addition to the nicotinic cholinergic and dopaminergic (DAergic) systems that function as fundamental targets [37, 38], an increasing number of studies suggest important roles of the endocannabinoid (ECB) system, which includes cannabinoid (CB) receptors such CB1 and CB2 receptors and the endogenous ligands for these receptors, in NC addiction [39, 40]. Molecularly, overlapping distributions of CB1 and nACh receptors in some brain regions, and functional interactions between these receptors have been reported [41, 42]. Coexpression of CB1 and dopamine (DA) receptors in distinct brain regions, as well as their functional interactions, has also been reported [43, 44]. Furthermore, modulation of the ECB system controls the nAChR-mediated DA release evoked by NC [45]. In rodents, NC-induced anxiety- and depression-related behaviors were modulated by CB ligands, although the effects were different depending on the condition of NC administration [46–50]. The crucial involvement of the ECB system has also been reported in stress-related emotional symptoms including anxiety and depression [51–53]. Epigenetically, the involvement of decreased histone acetylation has been reported in the repressed transcription of the striatal CB1 receptor gene in a mouse model of Huntington’s disease [54]. On the other hand, in the hippocampus and neocortex of neonatal mice, the involvement of ethanol-induced amplification of histone acetylation in the exon region of the CB1 receptor gene, which enhanced the function of CB1 receptors, has been reported in the memory-related neurobehavioral abnormalities after growth [55]. In the prefrontal cortex of adolescent rats, increased histone acetylation was induced by the CB1 agonist Δ(9)-tetrahydrocannabinol (THC) [56]. To date, however, epigenetic processes directly associated with the involvement of the ECB system in the effects of NC and/or stressors have not been demonstrated. Some experimental studies showed antagonistic effects of CB agonists against both histone modifications (phosphorylation or phosphoacetylation) and behavioral abnormalities (seizures or dyskinesias) mediated by the neurotransmitter systems related to NC and/or stressors (e.g. DAergic system) [57, 58]. Nevertheless, the role of histone acetylation, a representative epigenetic process implicated in the behavioral effects of NC and stressors [33–36], in the interacting effects of NC and/or stressors with the ECB system has not been investigated.
In the present study, using behavioral tests in mice (elevated plus-maze (EPM) and forced swimming (FS) test), anxiety- and depression-related behavioral alterations caused by NC and/or immobilization stress (IM), a typical stressor, were investigated, considering the epigenetic involvement of histone acetylation as previously reported [33–36, 59, 60]. Moreover, based on the above-suggested contributing roles of the ECB system and possibility of epigenetic involvement [54–58], the interacting influence of selected CB1 ligands on the effects of histone deacetylase (HDAC) inhibitors that mainly induce histone acetylation, possibly on the therapeutic effects, was evaluated.
Materials and Methods
Subjects and Ethics Statement
Based on previous studies on NC and stressor treatments [47, 50], male ICR mice (80 ± 10 days old) (Shizuoka Laboratory Animal Center, Hamamatsu, Japan) were housed in a forced-air facility, which was maintained at 23°C and 50% relative humidity, with a 12 h/12 h light/dark cycle. The mice were kept separately in single transparent cages measuring 23.5 × 16.5 × 12 cm, and were allowed water and rodent chow ad libitum. The experiments described in this report were approved by the Animal Care and Use Committees of Kyoto University, and were conducted in accordance with the “Regulation on Animal Experimentation at Kyoto University” of the institution (established in 2007 and updated in 2013) [61], which is based on the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All efforts were made by trained personnel in order to minimize the pain experienced by the mice. No mice died during the experiments. All of the observations and evaluations were performed by a trained observer who was blinded to and not informed of the treatment conditions in advance. Each experimental group contained 10 mice.
Drug and Stressor Treatments
The protocols for the NC and stressor treatments were determined based on preliminary experiments and previous studies [47, 50, 62]. With respect to NC, repeated subcutaneous (s.c.) doses of NC that caused the emotional behaviors (anxiety- and depression-like behaviors) most effectively in mice [50] were selected: a single s.c. dose of 0.8 mg/kg was administered daily for 4 days. NC (Nacalai Tesque, Inc., Kyoto, Japan) was supplied in free-base form at 95% purity, and was freshly dissolved in saline to a volume of 5 ml/kg immediately before each administration. With respect to the stressor, treatments using IM, which have also been demonstrated to cause these emotional behaviors in rodents [50, 63], were used. In the present experiments, repeated IM treatments in which the effects were almost equivalent to the peak effects of the NC treatments in preliminary experiments were selected: 10 min of IM, which was induced by placing the mouse in a narrow space (diameter about 12 cm) in a vinyl bag with some breathing holes, was performed once per day for 4 days. Furthermore, to investigate the interactions between NC and IM, the behavioral alterations were examined in the NC plus IM group (NC-IM group) which received the above s.c. dose of NC 10 min before the IM treatment once per day for 4 days, according to a previously reported study [64].
The HDAC inhibitors sodium butyrate (SB) and valproic acid (VA), the selective CB1 agonist ACPA (arachidonylcyclopropylamide; AC), and the selective CB1 antagonist SR 141716A (N-(Piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide hydrochloride; SR) were purchased from Tocris Cookson Inc. (Ellisville, Missouri, USA), and the doses were selected based on previous studies and preliminary experiments [65–70]. For each drug, the data were collected and shown for those intraperitoneal (i.p.) doses that induced no toxic behavioral alterations by themselves at the prescribed time point: 50, 100 and 200 mg/kg for SB, 200, 300 and 400 mg/kg for VA, 0.05, 0.2 and 1 mg/kg for AC, and 0.5, 1 and 2 mg/kg for SR. Furthermore, in the experiments examining the interacting role of HDAC inhibition (histone acetylation) with the ECB system, the CB1 antagonist SR was used in combination with the effective HDAC inhibitors, based on previous studies [66,68]. The drugs were dissolved and diluted using a mixed solution of dimethylsulphoxide (DMSO) plus distilled water, and were administered in a volume of 2.5 ml/kg 60 min (SB, AC and SR) or 30 min (VA) before each NC, IM or NC-IM treatment, based on previous data and preliminary experiments [65–70]. In the HDAC inhibitor- or CB1 ligand-only groups, equivolume saline vehicle was injected instead of the NC, IM or NC-IM treatment. In the control group without any drug or stressor treatment (control group), the mixed vehicle solution of DMSO and distilled water was injected instead of the CB1 ligands, and then equivolume saline vehicle was injected instead of the NC, IM or NC-IM treatment. The drug and stressor treatments and each experimental session were performed between 12 h and 16 h of the light cycle.
Behavioral Tests
Elevated plus-maze (EPM) test
Based on previous studies [46, 50, 71–73], alterations in anxiety-related behaviors were examined in the EPM test, using a cardboard apparatus that consisted of two opposite open arms 50 × 10 cm (length and width) and two enclosed arms 50 × 10 × 30 cm (length, width, and height), positioned 50 cm from the floor. After the number of entries into open arms, the time spent on open arms (sec), and the total number of entries into arms were evaluated (5 min test periods), the percentage of entries into open arms and the percentage of time spent on open arms were calculated as parameters of anxiety-related behaviors. The total number of entries into arms was assessed as a parameter representing locomotor activity [72]. Based on previous data [50], the evaluations of these parameters were performed at the 2 h time point after the last NC, IM or NC-IM treatment. At the beginning of each experimental session, each mouse was placed diagonally in the center platform of the maze, facing both the open and enclosed arms [50].
Forced swimming (FS) test
Based on previous studies [63, 74, 75], alterations in depression-related behaviors were examined in the FS test, using a glass cylinder apparatus 33 cm in height and 18 cm in diameter containing 14 cm of water at 21–23°C and the activity-measuring and recording system Supermex-CompACT AMS instrument (Muromachi Kikai Co. Ltd., Tokyo, Japan), for which an infrared sensor was placed over the cylinder at a distance of 20 cm from the water and the frequency of each mouse crossing the area under the sensor while swimming was measured as a number of counts. As parameters of the test, the time until immobility, that is, the time after when only modest swimming behaviors necessary to avoid drowning (<60 counts/min under the present conditions), and the activity counts (per 10 min) which reflected the amount of swimming behaviors during a 10 min experimental period were monitored. Considering the time course of the behavioral alterations [47], the evaluations of these parameters were performed at the 2 h time point after the last NC, IM or NC-IM treatment.
Statistical analysis
The data were subjected to two- or three-way analysis of variance (ANOVA) for each experiment. With respect to the experiments examining the NC- and/or IM-induced anxiety- and depression-related behavioral alterations and the effects of HDAC inhibitors or CB1 ligands, a 2 (NC versus vehicle) × 2 (IM versus vehicle) or 2 (NC versus vehicle) × 2 (IM versus vehicle) × 4 (three doses of each HDAC inhibitor or CB1 ligand versus vehicle) factorial design was used [76, 77]. With respect to the experiments examining the interacting role of HDAC inhibition (histone acetylation) with the ECB system, a 4 (NC, IM, NC-IM versus vehicle) × 2 (most effective dose of each HDAC inhibitor or CB1 ligand versus vehicle) × 4 (three doses of the CB1 antagonist SR versus vehicle) factorial design was used [76, 77]. For pairwise comparisons, Bonferroni post-hoc tests were performed [76]. All of the comparisons were performed using statistical software packages (“Excel Statistics” from Social Survey Research Information Co. Ltd., Tokyo, Japan). P values less than 0.05 were considered to be statistically significant.
Results
Antagonistic effects of HDAC inhibitors and CB1 agonist against NC- and/or IM-induced anxiety-like behavioral alterations in the elevated plus-maze (EPM) test
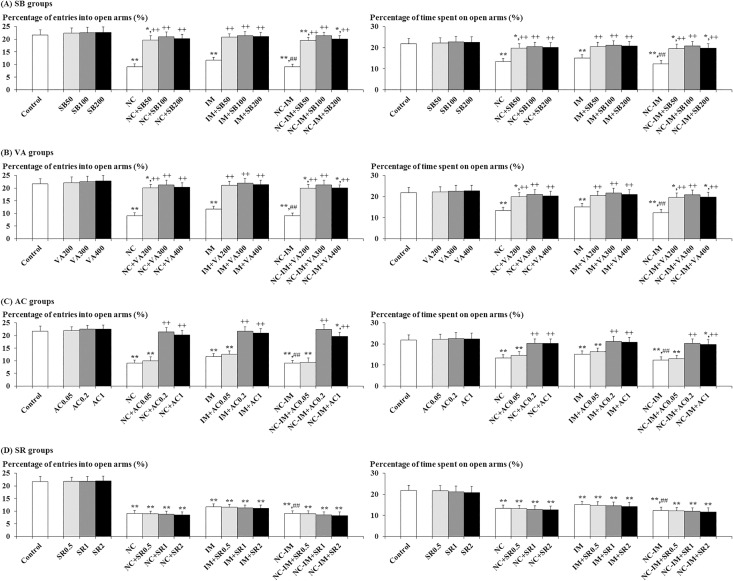
In both NC, IM and NC-IM groups, at the 2 h time point after the last treatment, anxiety-like behavioral alterations, i.e. statistically significantly attenuated percentage of entries into open arms and significantly attenuated percentage of time spent on open arms, were observed in the EPM test (Fig 1). This is consistent with the results of the ANOVA revealing statistically significant main effects of NC (F(1, 36) = 302.48, P<0.001 for the percentage of entries into open arms and F(1, 36) = 102.70, P<0.001 for the percentage of time spent on open arms) and IM (F(1, 36) = 131.82, P<0.001 for the percentage of entries into open arms and F(1, 36) = 50.58, P<0.001 for the percentage of time spent on open arms). For the NC-IM group, the parameter values were significantly attenuated as compared to the IM group, which is consistent with the results of the ANOVA revealing significant interactions between the NC and IM treatment for each parameter value (F(1, 36) = 133.23, P<0.001 for the percentage of entries into open arms and F(1, 36) = 25.38, P<0.001 for the percentage of time spent on open arms).
Fig 1. Antagonistic effects of histone deacetylase (HDAC) inhibitors or cannabinoid type 1 (CB1) agonist against anxiety-like behaviors.
The parameter values of the elevated plus-maze test at the 2 h time point after the last nicotine (NC) (0.8 mg/kg, s.c.) or immobilization stress (IM) (10 min) treatment are shown as means ± S.D. (n = 10) for each HDAC inhibitor or CB1 ligand co-treatment group (with each i.p. dose (mg/kg)). (A) Sodium butyrate (SB) co-treatment groups (SB groups); (B) Valproic acid (VA) co-treatment groups (VA groups); (C) ACPA (arachidonylcyclopropylamide; AC) co-treatment groups (AC groups); (D) SR 141716A (N-(Piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide hydrochloride; SR) co-treatment groups (SR groups). The data for the control, NC, IM, and NC plus IM (NC-IM) groups without any HDAC inhibitor or CB1 ligand co-treatments, as well as the HDAC inhibitor- and CB1 ligand-only groups, are also shown. * (p<0.05), ** (p<0.01): significant attenuation as compared to the control group; ++ (p<0.01): significant increase as compared to the NC, IM, or NC-IM group without any co-treatments; ## (p<0.01): significant attenuation as compared to the IM group without any co-treatments.
Against these anxiety-like behavioral alterations, statistically significant antagonistic effects, i.e. recoveries from both attenuated percentage of entries into open arms and attenuated percentage of time spent on open arms, were observed in both NC, IM and NC-IM groups co-treated with the HDAC inhibitor SB (50–200 mg/kg), VA (200–400 mg/kg) or the CB1 agonist AC (0.2–1 mg/kg) (Fig 1A–1C). This is consistent with the results of the ANOVA revealing statistically significant main effects of SB (F(3, 144) = 258.06, P<0.001 for the percentage of entries into open arms and F(3, 144) = 59.25, P<0.001 for the percentage of time spent on open arms), VA (F(3, 144) = 237.24, P<0.001 for the percentage of entries into open arms and F(3, 144) = 62.75, P<0.001 for the percentage of time spent on open arms), and AC (F(3, 144) = 374.26, P<0.001 for the percentage of entries into open arms and F(3, 144) = 71.28, P<0.001 for the percentage of time spent on open arms). Furthermore, significant interactions between all of the following treatments were observed: SB versus NC (F(3, 144) = 35.67, P<0.001 for the percentage of entries into open arms and F(3, 144) = 9.57, P<0.001 for the percentage of time spent on open arms), SB versus IM (F(3, 144) = 17.55, P<0.001 for the percentage of entries into open arms and F(3, 144) = 5.26, P<0.01 for the percentage of time spent on open arms), VA versus NC (F(3, 144) = 31.57, P<0.001 for the percentage of entries into open arms and F(3, 144) = 9.36, P<0.001 for the percentage of time spent on open arms), VA versus IM (F(3, 144) = 16.67, P<0.001 for the percentage of entries into open arms and F(3, 144) = 5.42, P<0.01 for the percentage of time spent on open arms), AC versus NC (F(3, 144) = 61.46, P<0.001 for the percentage of entries into open arms and F(3, 144) = 11.80, P<0.001 for the percentage of time spent on open arms), and AC versus IM (F(3, 144) = 28.57, P<0.001 for the percentage of entries into open arms and F(3, 144) = 6.51, P<0.001 for the percentage of time spent on open arms). In each group co-treated with the CB1 antagonist SR, as well as in each HDAC inhibitor- or CB1 ligand-only group, no significant alterations as compared to the control group were observed for each parameter value under the present experimental conditions.
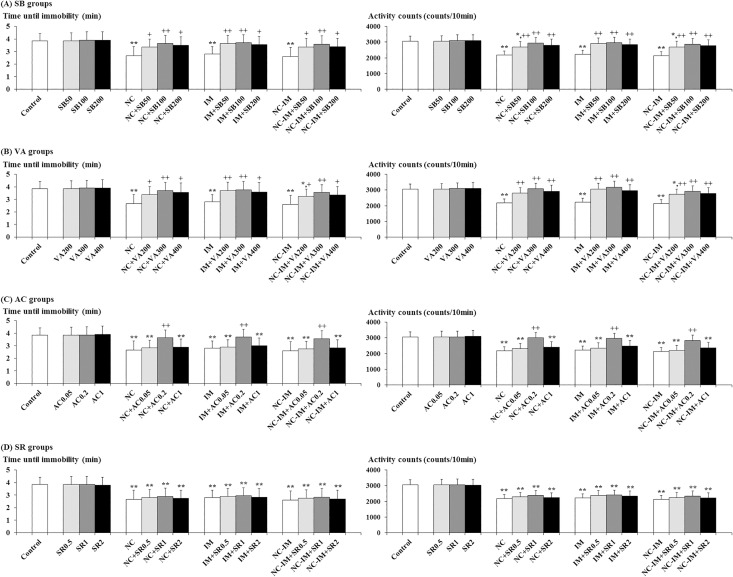
Antagonistic effects of HDAC inhibitors and CB1 agonist against NC- and/or IM-induced depression-like behavioral alterations in the forced swimming (FS) test
In both NC, IM and NC-IM groups, at the 2 h time point after the last treatment, depression-like behavioral alterations, i.e. statistically significantly attenuated time until immobility and significantly attenuated activity counts which reflected both the overall activity during the swim behaviors and the minimum activity after immobility, were observed in the FS test (Fig 2). This is consistent with the results of the ANOVA revealing statistically significant main effects of NC (F(1, 36) = 9.77, P<0.01 for the time until immobility and F(1, 36) = 29.84, P<0.001 for the activity counts) and IM (F(1, 36) = 6.03, P<0.05 for the time until immobility and F(1, 36) = 22.69, P<0.001 for the activity counts). For the NC-IM group, the parameter values were not significantly different from either NC- or IM-only group (Fig 2).
Fig 2. Antagonistic effects of histone deacetylase (HDAC) inhibitors or cannabinoid type 1 (CB1) agonist against depression-like behaviors.
The parameter values of the forced swimming test at the 2 h time point after the last nicotine (NC) (0.8 mg/kg, s.c.) or immobilization stress (IM) (10 min) treatment are shown as means ± S.D. (n = 10) for each HDAC inhibitor or CB1 ligand co-treatment group (with each i.p. dose (mg/kg)). (A) Sodium butyrate (SB) co-treatment groups (SB groups); (B) Valproic acid (VA) co-treatment groups (VA groups); (C) ACPA (arachidonylcyclopropylamide; AC) co-treatment groups (AC groups); (D) SR 141716A (N-(Piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide hydrochloride; SR) co-treatment groups (SR groups). The data for the control, NC, IM, and NC plus IM (NC-IM) groups without any HDAC inhibitor or CB1 ligand co-treatments, as well as the HDAC inhibitor- and CB1 ligand-only groups, are also shown. * (p<0.05), ** (p<0.01): significant attenuation as compared to the control group; + (p<0.05), ++ (p<0.01): significant increase as compared to the NC, IM, or NC-IM group without any co-treatments.
Against these depression-like behavioral alterations, statistically significant antagonistic effects, i.e. recoveries from both attenuated time until immobility and attenuated activity counts, were observed in both NC, IM and NC-IM groups co-treated with SB (50–200 mg/kg), VA (200–400 mg/kg) or AC (0.2 mg/kg) (Fig 2A–2C). This is consistent with the results of the ANOVA revealing statistically significant main effects of SB (F(3, 144) = 8.92, P<0.001 for the time until immobility and F(3, 144) = 20.03, P<0.001 for the activity counts), VA (F(3, 144) = 9.12, P<0.001 for the time until immobility and F(3, 144) = 26.35, P<0.001 for the activity counts), and AC (F(3, 144) = 8.86, P<0.001 for the time until immobility and F(3, 144) = 20.83, P<0.001 for the activity counts). In each group co-treated with the CB1 antagonist SR, as well as in each HDAC inhibitor- or CB1 ligand-only group, no significant alterations as compared to the control group were observed for each parameter value under the present experimental conditions.
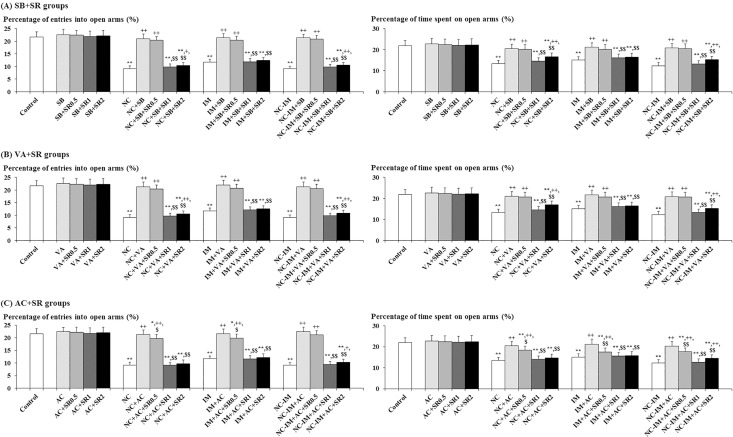
Interacting effects between HDAC inhibitors and CB1 antagonist
In order to investigate the interacting role of HDAC inhibitors with the ECB system, interactions with the CB1 antagonist SR (0.5, 1 and 2 mg/kg) were examined for the most effective dose of the HDAC inhibitors SB (100 mg/kg) and VA (300 mg/kg). For comparison, interactions with the same doses of SR were examined for the most effective dose of the CB1 agonist AC (0.2 mg/kg).
Against the anxiolytic-like effects of SB and VA in the EPM test, as well as against those effects of AC, significant antagonistic effects were provided by SR (1 and 2 mg/kg) for each parameter in both NC, IM and NC-IM groups (Fig 3). These data are consistent with the results of ANOVA revealing statistically significant interactions of the following treatments: NC and/or IM × SB × SR (F(9, 288) = 16.53, P<0.001 for the percentage of entries into open arms and F(9, 288) = 3.10, P<0.01 for the percentage of time spent on open arms), NC and/or IM × VA × SR (F(9, 288) = 15.16, P<0.001 for the percentage of entries into open arms and F(9, 288) = 3.25, P<0.001 for the percentage of time spent on open arms), and NC and/or IM × AC × SR (F(9, 288) = 17.69, P<0.001 for the percentage of entries into open arms and F(9, 288) = 2.93, P<0.01 for the percentage of time spent on open arms).
Fig 3. Interacting effects between cannabinoid type 1 (CB1) antagonist (SR 141716A) and efficacious (anxiolytic-like) histone deacetylase (HDAC) inhibitors or CB1 agonist against anxiety-like behavioral alterations caused by nicotine (NC) and/or immobilization stress (IM).
The parameter values of the elevated plus-maze test at the 2 h time point after the last NC (0.8 mg/kg, s.c.) or IM (10 min) treatment are shown as means ± S.D. (n = 10) for each HDAC inhibitor or CB1 ligand “plus” SR 141716A (N-(Piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide hydrochloride; SR) co-treatment group (with each i.p. dose (mg/kg)). (A) Sodium butyrate (SB) plus SR co-treatment groups (SB+SR groups); (B) Valproic acid (VA) plus SR co-treatment groups (VA+SR groups); (C) ACPA (arachidonylcyclopropylamide; AC) plus SR co-treatment groups (AC+SR groups). The data for the control, NC, IM, and NC plus IM (NC-IM) groups without any HDAC inhibitor or CB1 ligand co-treatments, as well as the HDAC inhibitor-, CB1 agonist-, HDAC inhibitor plus SR-, and CB1 agonist plus SR-only groups, are also shown. * (p<0.05), ** (p<0.01): significant attenuation as compared to the control group; + (p<0.05), ++ (p<0.01): significant increase as compared to the NC, IM, or NC-IM group without any co-treatments; $ (p < 0.05), $ $ (p < 0.01): significant attenuation as compared to the NC, IM, or NC-IM group co-treated with the efficacious HDAC inhibitor or CB1 agonist.
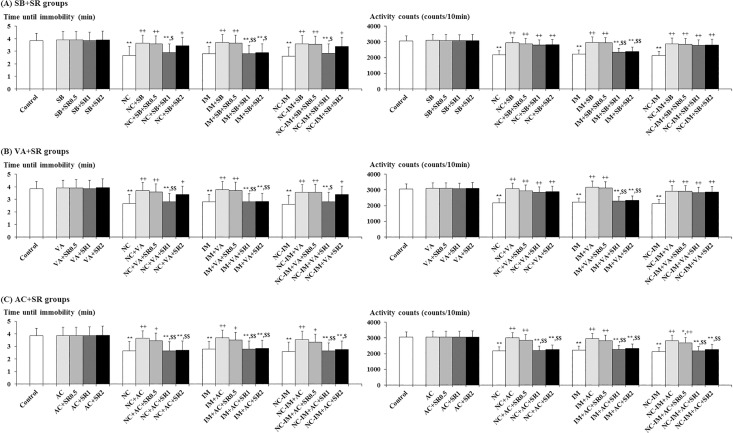
Against the antidepressant-like effects of SB and VA in the FS test, significant antagonistic effects were provided by SR (1 and 2 mg/kg) for each parameter in the IM groups (Fig 4A and 4B), which is consistent with the results of the ANOVA revealing statistically significant interactions of treatments for both SB versus SR (F(3, 288) = 4.67, P<0.01 for the time until immobility and F(3, 288) = 4.28, P<0.01 for the activity counts) and VA versus SR (F(3, 288) = 5.42, P<0.01 for the time until immobility and F(3, 288) = 6.75, P<0.001 for the activity counts). However, in the NC and NC-IM groups, only limited antagonistic effects (i.e. significant antagonistic effects only against recovered time until immobility) were provided by 1 mg/kg SR against the effects of SB and VA (Fig 4A and 4B). On the other hand, against the antidepressant-like effects of AC, significant antagonistic effects were provided by SR (1 and 2 mg/kg) for each parameter in both NC, IM and NC-IM groups (Fig 4C), which is consistent with the results of the ANOVA revealing statistically significant interactions of treatments for AC versus SR (F(3, 288) = 6.52, P<0.001 for the time until immobility and F(3, 288) = 17.37, P<0.001 for the activity counts).
Fig 4. Interacting effects between cannabinoid type 1 (CB1) antagonist (SR 141716A) and efficacious (antidepressant-like) histone deacetylase (HDAC) inhibitors or CB1 agonist against depression-like behavioral alterations caused by nicotine (NC) and/or immobilization stress (IM).
The parameter values of the forced swimming test at the 2 h time point after the last NC (0.8 mg/kg, s.c.) or IM (10 min) treatment are shown as means ± S.D. (n = 10) for each HDAC inhibitor or CB1 ligand “plus” SR 141716A (N-(Piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide hydrochloride; SR) co-treatment group (with each i.p. dose (mg/kg)). (A) Sodium butyrate (SB) plus SR co-treatment groups (SB+SR groups); (B) Valproic acid (VA) plus SR co-treatment groups (VA+SR groups); (C) ACPA (arachidonylcyclopropylamide; AC) plus SR co-treatment groups (AC+SR groups). The data for the control, NC, IM, and NC plus IM (NC-IM) groups without any HDAC inhibitor or CB1 ligand co-treatments, as well as the HDAC inhibitor-, CB1 agonist-, HDAC inhibitor plus SR-, and CB1 agonist plus SR-only groups, are also shown. * (p<0.05), ** (p<0.01): significant attenuation as compared to the control group; + (p<0.05), ++ (p<0.01): significant increase as compared to the NC, IM, or NC-IM group without any co-treatments; $ (p < 0.05), $ $ (p < 0.01): significant attenuation as compared to the NC, IM, or NC-IM group co-treated with the efficacious HDAC inhibitor or CB1 agonist.
Discussion
NC- and/or IM-induced anxiety- and depression-like behavioral alterations and antagonistic effects of HDAC inhibitors
In the NC group receiving repeated treatments of 0.8 mg/kg NC, as well as in the IM group, anxiety- and depression-like behavioral alterations were observed in the EPM and FS tests under the present experimental conditions (Figs 1 and 2), which supports the data in previous studies [47, 50, 63]. Although the opposite effects on anxiety and depression have been reported for NC depending on the experimental condition [3, 13–17, 46–50, 78], anxiogenic- and depressogenic-like effects like those observed with the IM treatment were observed with the NC treatment in the present study. In the author’s preliminary experiments, consistent with several previous studies [25, 47, 50, 63, 75, 78, 79], even acute 1-day treatment of NC or IM induced both anxiety- and depression-like behaviors in mice. However, with the repeated 4-day treatment used in the present study, enhanced anxiety- and depression-like behaviors as compared to the acute 1-day treatment were observed at the selected time point (2 h) after the last treatment. Yet, this mouse model of subacute treatment with a small number of repeated doses did not seem to sufficiently reflect the human cases of daily and/or dependent smoking who are suffering from anxiety and depression [5–10]. Nevertheless, since recent studies have reported that some brain dysfunction is caused by small amounts of NC intake even in non-smoking humans [80–82], it is possible that the present results on NC-induced anxiety and depression in mice mimic some kind of latent negative influence on emotion-related brain function in humans.
As reviewed previously, the involvement of stress-related neurotransmitter systems, such as the DAergic and serotonergic systems (DA and serotonin receptors), has been reported in the development of anxiety and depression: neuroimaging and pharmacological studies have demonstrated that the dysfunction of DA and serotonin receptors is associated with increased anxiety and depression [83–86]. Furthermore, the combined influence of the nicotinic cholinergic system (nAChRs) and DAergic system, both of which also function as targets for NC, was closely correlated with the elicitation of anxiety- and depression-like behavioral responses [87–89]. The nicotinic cholinergic system also cooperated with the serotonergic system in modulating anxiety and depression [88, 90, 91]. In addition to the neurotransmitter systems, several stress-related neuromolecular responses, such as those of the neuroendocrine system (e.g. secretion of hypothalamic-pituitary-adrenal (HPA)-axis hormones such as corticosterone, norepinephrine, etc.), immediate early gene (IEG) (e.g. Arc, c-Fos, etc.) expression and dysregulated hippocampal neurogenesis, have been reported to accompany and participate in the control of anxiety- and depression-like behaviors, in which the involvement of the DAergic and serotonergic systems, as well as the nicotinic cholinergic system, has been suggested [92–104]. Similar modifications in these neuromolecular responses were also induced by NC in some rodent experimental models [105–107]. Moreover, as discussed later, these neuromolecular responses and the modulation of the stress-related neurotransmitter systems were associated with epigenetic histone acetylation.
With respect to interactions between NC and IM in the NC-IM group, statistically significant enhancement of anxiogenic-like effects was caused by NC plus IM as compared to the IM-only group in the EPM test (Fig 1). In the FS test, no significant alterations in depressogenic-like effects were observed (Fig 2). While the relationship between stressors such as IM and NC in the behavioral effects is controversial and “antistress” effects of pre-exposed NC (cigarette) have also been reported depending on the condition [24–26], significant synergistic effects like those observed in previous studies [21–23] were provided by the IM plus NC treatment in the present anxiety-related experimental model. An augmented increase in secreted HPA-axis hormones and immediate early gene expression, which accompanied the enhanced behavioral effects, has also been reported in several previous studies [108, 109]. Nevertheless, in the depression-related behavioral test (FS test), no significant interacting effects between IM and NC were observed for the behavioral alterations. Although the molecular mechanisms underlying these differences in NC/IM interactions between anxiety- and depression-related behavioral alterations were not clarified, decreased interacting effects of stressors against NC-induced behaviors have been reported depending on the type of NC and/or stressor treatment, assessed type of behavioral response and genotype [110–112]. The blunted interacting effects for neuromolecular responses were also suggested depending on the experimental condition [110–112]. Moreover, considering the additional interactions with the HDAC inhibitors (Figs 1 and 2) and putative influence of histone acetylation in the NC-IM group, there is a possibility that the incongruous interactions between NC and IM in the present study were closely associated with multiple molecular modifications at the epigenetic level.
The NC- and/or IM-induced anxiety- and depression-like behavioral alterations were antagonized by the HDAC inhibitors SB and VA (Figs 1 and 2). In previous studies, anxiogenic- and depressogenic-like effects of various stressors were antagonized by SB and VA [60, 67, 113, 114]. From the present results, the epigenetic involvement of histone acetylation in the elicitation of NC-induced anxiety- and depression-like behaviors was also suggested. Molecularly, decreased histone acetylation induced in some brain regions (e.g. hippocampus and nucleus accumbens) by stressors was involved in the dysregulation of the stress-related neurotransmitter systems such as the DAergic and serotonergic systems (e.g. decreased synthesis of DA and serotonin), as well as anxiety- and depression-like behavioral alterations, and HDAC inhibitors regulatorily antagonized both molecular and behavioral effects [115, 116]. Although the contribution of histone acetylation to the modulated function of the direct NC target nAChRs has not been fully elucidated, reduced histone acetylation at the promoters of the gene encoding the acetylcholine-hydrolyzing enzyme acetylcholinesterase (AChE), which seemed to dysregulate the function of the nicotinic cholinergic system, has been reported in the hippocampus of stressor-treated mice eliciting anxiety-like behaviors, and HDAC inhibition mediated by gene manipulation regulatorily abolished both AChE-related effects and stress-related anxiety [117]. Moreover, in rats treated with repeated NC, increased histone acetylation was observed at several promoters of the gene encoding DA receptors, which function as NC target receptors, resulting in increased expression of the DA receptor gene in the prefrontal cortex, one of the target areas for anxiolytics and antidepressants that is also closely associated with the nicotinic cholinergic system [118]. Furthermore, the involvement of histone acetylation in the above-mentioned stress-related responses such as modulated secretion of HPA-axis hormones, IEG expression, and hippocampal neurogenesis has been reported, and accompanying modulation of anxiety- and depression-related behavioral symptoms has been suggested [119–125]. Although the influence of histone acetylation varied depending on the type and duration of stressors, decreased acetylation was directly induced by stressors in several experimental models [124, 125], and thus “anti-stress” effects of increased acetylation provided by HDAC inhibitors were predicted. In the present study, the antagonistic effects of the HDAC inhibitors SB and VA against the stressor (including NC)-induced anxiety- and depression-like behaviors were not dose-dependent. The mechanism underlying this dose-response has not been clarified, but seemed to be correlated with the attenuated anti-stress effects reported for high doses of the HDAC inhibitors, and the modulated involvement of related neurotransmitter systems (e.g. DAergic, GABAergic and glutamatergic systems) was suggested [123, 126].
Effects of ECB ligands against NC- and/or IM-induced anxiety- and depression-like behavioral alterations and putative epigenetic interactions with HDAC inhibitors
In the present experimental model, the selective CB1 agonist AC antagonized the NC- and/or IM-induced anxiety- and depression-like behavioral alterations (Figs 1 and 2). These results were predictable considering the above-mentioned potent controlling roles of the ECB system in the effects of NC and stressors [39, 40, 51–53], and were consistent with the central CB1 agonist-induced anxiolytic- and antidepressant-like effects provided against both NC- and stressor-induced behavioral alterations in a number of previous rodent studies [46, 48, 50, 127–130]. Neuroanatomical overlap and functional interactions between the ECB system (CB1 receptors) and NC- and/or stress-related neurotransmitter systems (e.g. nicotinic cholinergic, DAergic and serotonergic systems) also support the present results [41–45, 52, 53, 131]. Furthermore, the activation of the ECB system was involved in the attenuation of the stress-related neuromolecular responses accompanying anxiety- and depression-like behaviors (e.g. responses of the neuroendocrine system, c-fos expression and decreased hippocampal neurogenesis), which seemed to be associated with epigenetic histone acetylation [51, 132–135]. In the present study, obvious dose-dependent effects were not observed for CB1 agonists, and seemed to be due to the mechanisms related to multiple neurotransmitter systems including both ECB and stress-related neurotransmitter systems involved in anti-anxiety and/or anti-depression (e.g. GABAergic, glutamatergic and serotonergic systems) [129, 130, 136, 137]. In previous studies, depending on the treatment condition, the anxiogenic- and depressogenic-like effects of NC or stressors were enhanced or accompanied by the activation of the ECB system and antagonized by CB antagonists such as SR, in which the varying involvement of the relevant neurotransmitter systems such as GABAergic, glutamatergic and serotonergic systems, in addition to the ECB system, has been suggested [127, 130, 137–143]. However, under the present condition, anxiolytic- and antidepressant-like effects were elicited by the CB1 agonist AC, and these effects were blocked by the co-administration of the CB1 antagonist SR (Figs 3 and 4). Although the non-toxic doses of SR selected in the present study were behaviorally inactive against the NC- and/or IM-induced anxiogenic- and depressogenic-like effects, the blocking effects against AC were provided by each dose. The effects of SR were not dose-dependent and seemed to be correlated with the complicated modulation of the neurotransmitter systems other than the ECB system (e.g. GABAergic and catecholaminergic systems), as previously reported [130, 144, 145].
Like the effects induced by AC, the anxiolytic- and antidepressant-like effects elicited by the HDAC inhibitors SB and VA were to some extent blocked by the co-administration of the CB1 antagonist SR (Figs 3 and 4). From these results, it could be predicted that epigenetic histone acetylation has an important role in both activation of the ECB system and anxiolytic/antidepressant-like behavioral responses. To date, several studies mentioned above investigated the role of histone acetylation in the modulated function of the ECB system (CB1 receptors): increased function of the ECB system was accompanied by increased histone acetylation in the normal brain, but the histone acetylation-induced increase in the expression of CB1 receptors occurred in some pathological (stress-loaded) conditions such as neonatal ethanol treatment [54–56]. Moreover, there have been reports on the contribution of excess histone acetylation-related enhanced function of the ECB system during the neonatal period to impaired memory-related behavioral alterations in adulthood [55], but the involvement of such epigenetically modulated function of the ECB system (CB1 receptors) in the anxiolytic- and antidepressant-like effects induced by HDAC inhibitors has not been examined. With respect to other types of histone modifications (phosphorylation or phosphoacetylation) related to HDAC inhibitors, the association with both function of the ECB system modulated by CB agonists and behavioral alterations involving the stress-related neurotransmitter system (i.e. seizures or dyskinesias related to the DAergic system) has been reported, as mentioned above [57, 58]. Furthermore, based on the present results that some blocking influence was provided by the CB1 antagonist SR against the SB- and VA-induced anxiolytic- and antidepressant-like effects, as well as against the CB1 agonist AC-induced attenuating effects on anxiety and depression, the HDAC inhibitor-induced histone acetylation seemed to contribute to the activation of the ECB system (CB1 receptors), at least indirectly through the modulation of some relevant neurotransmitter systems, and then play an important role in the attenuation of NC- and/or stressor-induced anxiety- and depression-like behaviors. Therefore, some molecular regulatory role of HDAC inhibitor-induced histone acetylation could be hypothesized in both function of the ECB system and stress/emotion-related behaviors, although direct evidence has not yet been demonstrated. In the NC treatment groups (NC and NC-IM groups), the antidepressant-like effects of the HDAC inhibitors SB and VA were antagonized to a limited degree by SR, and only the recovered time until immobility was impacted. It is possible that histone acetylation within the NC-related neurotransmitter systems other than the ECB system (e.g. nACh system) contributed more effectively to the antidepressant-like effects of SB and VA than to the anxiolytic-like effects.
In summary, the present results using HDAC inhibitors (SB and VA) show the involvement of epigenetic histone acetylation in the attenuation of NC- and/or IM-induced anxiety- and depression-like behavioral alterations. The selective CB1 agonist AC, like HDAC inhibitors, provided anxiolytic- and antidepressant-like effects against these behavioral alterations, which suggests the involvement of the ECB system, and the selective CB1 antagonist SR antagonized the effects of AC. Some attenuating influence of SR was also observed on the anxiolytic- and antidepressant-like effects of HDAC inhibitors. From the present results, it could be hypothesized that the HDAC inhibitor-induced histone acetylation was, at least to some extent, simultaneously associated with both function of the ECB system (CB1 receptors), one of the targets for HDAC inhibitors, and stressor (NC- and/or IM)-induced emotion-related (anxiety- and depression-like) behaviors. However, against the antidepressant-like effects of HDAC inhibitors, the attenuating influence of SR was limited in the NC treatment groups (NC and NC-IM groups). In the elicitation of HDAC inhibitor-induced antidepressant-like effects in the NC treatment groups, there may be varying involvement of histone acetylation within the ECB vs. other NC-related neurotransmitter systems, and important therapeutic roles of neurotransmitter systems other than the ECB system at the epigenetic level were also suggested.
Acknowledgments
The author thanks the staffs of Shimizu Laboratory Supplies Co. Ltd. for the technical assistance. The author also thanks Dr. Yoshiko Yamamoto and Dr. Keiichi Yamamoto, Yamamoto Research Institute of Legal Medicine, for the advice related to the data analysis.
Data Availability
All relevant data are within the paper.
Funding Statement
The author has no support or funding to report.
References
- 1.WHO. WHO report on the global tobacco epidemic. 2011. Available: http://www.who.int/tobacco/global_report/2011/en/.Accessed 25June 2015.
- 2.WHO. WHO report on the global tobacco epidemic. 2013. Available: http://www.who.int/tobacco/global_report/2013/en/.Accessed 25June 2015.
- 3.Picciotto MR, Brunzell DH, Caldarone BJ. Effect of nicotine and nicotinic receptors on anxiety and depression. Neuroreport. 2002;13:1097–1106. 10.1097/00001756-200207020-00006 [DOI] [PubMed] [Google Scholar]
- 4.Bruijnzeel AW. Tobacco addiction and the dysregulation of brain stress systems. Neurosci Biobehav Rev. 2012;36:1418–1441. 10.1016/j.neubiorev.2012.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hughes JR. Effects of abstinence from tobacco: valid symptoms and time course. Nicotine Tob Res. 2007;9:315–327. 10.1080/14622200701188919 [DOI] [PubMed] [Google Scholar]
- 6.Edwards AC, Kendler KS. Nicotine withdrawal-induced negative affect is a function of nicotine dependence and not liability to depression or anxiety. Nicotine Tob Res. 2011;13:677–685. 10.1093/ntr/ntr058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leventhal AM, Ameringer KJ, Osborn E, Zvolensky MJ, Langdon KJ. Anxiety and depressive symptoms and affective patterns of tobacco withdrawal. Drug Alcohol Depend. 2013;133:324–329. 10.1016/j.drugalcdep.2013.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.West R, Hajek P. What happens to anxiety levels on giving up smoking? Am J Psychiatry. 1997;154:1589–1592. 10.1176/ajp.154.11.1589 [DOI] [PubMed] [Google Scholar]
- 9.Parrott AC. Heightened stress and depression follow cigarette smoking. Psychol Rep. 2004;94:33–34. 10.2466/pr0.94.1.33-34 [DOI] [PubMed] [Google Scholar]
- 10.Taylor G, McNeill A, Girling A, Farley A, Lindson-Hawley N, Aveyard P. Change in mental health after smoking cessation: systematic review and meta-analysis. BMJ. 2014;348:g1151 10.1136/bmj.g1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Picciotto MR, Addy NA, Mineur YS, Brunzell DH. It is not “either/or”: activation and desensitization of nicotinic acetylcholine receptors both contribute to behaviors related to nicotine addiction and mood. Prog Neurobiol. 2008;84:329–342. 10.1016/j.pneurobio.2007.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson SM, Brunzell DH. Low dose nicotine and antagonism of β2 subunit containing nicotinic acetylcholine receptors have similar effects on affective behavior in mice. PLoS One. 2012;7:e48665 10.1371/journal.pone.0048665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Popik P, Krawczyk M, Kos T, Nalepa I, Kowalska M, Witarski T, et al. Nicotine produces antidepressant-like actions: Behavioral and neurochemical evidence. Eur J Pharmacol. 2005;515:128–133. 10.1016/j.ejphar.2005.04.009 [DOI] [PubMed] [Google Scholar]
- 14.Suemaru K, Yasuda K, Cui R, Li B, Umeda K, Amano M, et al. Antidepressant-like action of nicotine in forced swimming test and brain serotonin in mice. Physiol Behav. 2006;88:545–549. 10.1016/j.physbeh.2006.05.007 [DOI] [PubMed] [Google Scholar]
- 15.Andreasen JT, Redrobe JP. Antidepressant-like effects of nicotine and mecamylamine in the mouse forced swim and tail suspension tests: role of strain, test and sex. Behav Pharmacol. 2009;20:286–295. 10.1097/FBP.0b013e32832c713e [DOI] [PubMed] [Google Scholar]
- 16.Cohen A, Young RW, Velazquez MA, Groysman M, Noorbehesht K, Ben-Shahar OM, et al. Anxiolytic effects of nicotine in a rodent test of approach-avoidance conflict. Psychopharmacology (Berl). 2009;204:541–549. 10.1007/s00213-009-1486-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turner JR, Castellano LM, Blendy JA. Parallel anxiolytic-like effects and upregulation of neuronal nicotinic acetylcholine receptors following chronic nicotine and varenicline. Nicotine Tob Res. 2011;13:41–46. 10.1093/ntr/ntq206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Faravelli C, Lo Sauro C, Lelli L, Pietrini F, Lazzeretti L, Godini L, et al. The role of life events and HPA axis in anxiety disorders: a review. Curr Pharm Des. 2012;18:5663–5674. 10.2174/138161212803530907 [DOI] [PubMed] [Google Scholar]
- 19.Gold PW. The organization of the stress system and its dysregulation in depressive illness. Mol Psychiatry. 2015;20:32–47. 10.1038/mp.2014.163 [DOI] [PubMed] [Google Scholar]
- 20.Richards JM, Stipelman BA, Bornovalova MA, Daughters SB, Sinha R, Lejuez CW. Biological mechanisms underlying the relationship between stress and smoking: state of the science and directions for future work. Biol Psychol. 2011;88:1–12. 10.1016/j.biopsycho.2011.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aronson KR, Almeida DM, Stawski RS, Klein LC, Kozlowski LT. Smoking is associated with worse mood on stressful days: results from a national diary study. Ann Behav Med. 2008;36:259–269. 10.1007/s12160-008-9068-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Childs E, de Wit H. Hormonal, cardiovascular, and subjective responses to acute stress in smokers. Psychopharmacology (Berl). 2009;203:1–12. 10.1007/s00213-008-1359-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kotlyar M, Drone D, Thuras P, Hatsukami DK, Brauer L, Adson DE, et al. Effect of stress and bupropion on craving, withdrawal symptoms, and mood in smokers. Nicotine Tob Res. 2011;13:492–497. 10.1093/ntr/ntr011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi D, Ota S, Watanuki S. Does cigarette smoking relieve stress? Evidence from the event-related potential (ERP). Int J Psychophysiol. 2015;98:470–476. 10.1016/j.ijpsycho.2015.10.005 [DOI] [PubMed] [Google Scholar]
- 25.Hsu HR, Chen TY, Chan MH, Chen HH. Acute effects of nicotine on restraint stress-induced anxiety-like behavior, c-Fos expression, and corticosterone release in mice. Eur J Pharmacol. 2007;566:124–131. 10.1016/j.ejphar.2007.03.040 [DOI] [PubMed] [Google Scholar]
- 26.Andreasen JT, Henningsen K, Bate S, Christiansen S, Wiborg O. Nicotine reverses anhedonic-like response and cognitive impairment in the rat chronic mild stress model of depression: comparison with sertraline. J Psychopharmacol. 2011;25:1134–1141. 10.1177/0269881110391831 [DOI] [PubMed] [Google Scholar]
- 27.Waddington CH. The epigenotype. 1942. Int J Epidemiol. 2012;41:10–13. 10.1093/ije/dyr184 [DOI] [PubMed] [Google Scholar]
- 28.Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet. 2003;33 Suppl:245–254. 10.1038/ng1089 [DOI] [PubMed] [Google Scholar]
- 29.Wong CC, Mill J, Fernandes C. Drugs and addiction: an introduction to epigenetics. Addiction. 2011;106:480–489. 10.1111/j.1360-0443.2010.03321.x [DOI] [PubMed] [Google Scholar]
- 30.Nestler EJ. Epigenetic mechanisms of drug addiction. Neuropharmacology. 2014;76 Pt B:259–268. 10.1016/j.neuropharm.2013.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmidt HD, McGinty JF, West AE, Sadri-Vakili G. Epigenetics and psychostimulant addiction. Cold Spring Harb Perspect Med. 2013;3:a012047 10.1101/cshperspect.a012047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.D’Addario C, Francesco AD, Pucci M, Agrò AF, Maccarrone M. Epigenetic mechanisms and endocannabinoid signalling. FEBS J. 2013;280:1905–1917. 10.1111/febs.12125 [DOI] [PubMed] [Google Scholar]
- 33.Pastor V, Host L, Zwiller J, Bernabeu R. Histone deacetylase inhibition decreases preference without affecting aversion for nicotine. J Neurochem. 2011;116:636–645. 10.1111/j.1471-4159.2010.07149.x [DOI] [PubMed] [Google Scholar]
- 34.Castino MR, Cornish JL, Clemens KJ. Inhibition of histone deacetylases facilitates extinction and attenuates reinstatement of nicotine self-administration in rats. PLoS One. 2015;10:e0124796 10.1371/journal.pone.0124796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McEwen BS, Eiland L, Hunter RG, Miller MM. Stress and anxiety: structural plasticity and epigenetic regulation as a consequence of stress. Neuropharmacology. 2012;62:3–12. 10.1016/j.neuropharm.2011.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vialou V, Feng J, Robison AJ, Nestler EJ. Epigenetic mechanisms of depression and antidepressant action. Annu Rev Pharmacol Toxicol. 2013;53:59–87. 10.1146/annurev-pharmtox-010611-134540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clarke PB. Mesolimbic dopamine activation—the key to nicotine reinforcement? Ciba Found Symp. 1990;152:153–162. 10.1002/9780470513965.ch9 [DOI] [PubMed] [Google Scholar]
- 38.Stolerman IP, Shoaib M. The neurobiology of tobacco addiction. Trends Pharmacol Sci. 1991;12:467–473. 10.1016/0165-6147(91)90638-9 [DOI] [PubMed] [Google Scholar]
- 39.Maldonado R, Valverde O, Berrendero F. Involvement of the endocannabinoid system in drug addiction. Trends Neurosci. 2006;29:225–232. 10.1016/j.tins.2006.01.008 [DOI] [PubMed] [Google Scholar]
- 40.Scherma M, Fadda P, Le Foll B, Forget B, Fratta W, Goldberg SR, et al. The endocannabinoid system: a new molecular target for the treatment of tobacco addiction. CNS Neurol Disord Drug Targets. 2008;7:468–481. 10.2174/187152708786927859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Picciotto MR, Caldarone BJ, King SL, Zachariou V. Nicotinic receptors in the brain. Links between molecular biology and behavior. Neuropsychopharmacology. 2000;22:451–465. 10.1016/S0893-133X(99)00146-3 [DOI] [PubMed] [Google Scholar]
- 42.Viveros MP, Marco EM, Llorente R, Lamota L. The role of the hippocampus in mediating emotional responses to nicotine and cannabinoids: a possible neural substrate for functional interactions. Behav Pharmacol. 2007;18:375–389. 10.1097/FBP.0b013e3282d28fb4 [DOI] [PubMed] [Google Scholar]
- 43.Hermann H, Marsicano G, Lutz B. Coexpression of the cannabinoid receptor type 1 with dopamine and serotonin receptors in distinct neuronal subpopulations of the adult mouse forebrain. Neuroscience. 2002;109:451–460. 10.1016/S0306-4522(01)00509-7 [DOI] [PubMed] [Google Scholar]
- 44.Terzian AL, Drago F, Wotjak CT, Micale V. The Dopamine and Cannabinoid Interaction in the Modulation of Emotions and Cognition: Assessing the Role of Cannabinoid CB1 Receptor in Neurons Expressing Dopamine D1 Receptors. Front Behav Neurosci. 2011;5:49 10.3389/fnbeh.2011.00049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Melis M, Pillolla G, Luchicchi A, Muntoni AL, Yasar S, Goldberg SR, et al. Endogenous fatty acid ethanolamides suppress nicotine-induced activation of mesolimbic dopamine neurons through nuclear receptors. J Neurosci. 2008;28:13985–13994. 10.1523/JNEUROSCI.3221-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Balerio GN, Aso E, Maldonado R. Role of the cannabinoid system in the effects induced by nicotine on anxiety-like behaviour in mice. Psychopharmacology (Berl). 2006;184:504–513. 10.1007/s00213-005-0251-9 [DOI] [PubMed] [Google Scholar]
- 47.Hayase T. Chronologically overlapping occurrences of nicotine-induced anxiety- and depression-related behavioral symptoms: effects of anxiolytic and cannabinoid drugs. BMC Neurosci. 2007;8:76 10.1186/1471-2202-8-76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mannucci C, Navarra M, Pieratti A, Russo GA, Caputi AP, Calapai G. Interactions between endocannabinoid and serotonergic systems in mood disorders caused by nicotine withdrawal. Nicotine Tob Res. 2011;13:239–247. 10.1093/ntr/ntq242 [DOI] [PubMed] [Google Scholar]
- 49.Cippitelli A, Astarita G, Duranti A, Caprioli G, Ubaldi M, Stopponi S, et al. Endocannabinoid regulation of acute and protracted nicotine withdrawal: effect of FAAH inhibition. PLoS One. 2011;6:e28142 10.1371/journal.pone.0028142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hayase T. Working memory- and anxiety-related behavioral effects of repeated nicotine as a stressor: the role of cannabinoid receptors. BMC Neurosci. 2013;14:20 10.1186/1471-2202-14-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Riebe CJ, Wotjak CT. Endocannabinoids and stress. Stress. 2011;14:384–397. 10.3109/10253890.2011.586753 [DOI] [PubMed] [Google Scholar]
- 52.McLaughlin RJ, Gobbi G. Cannabinoids and emotionality: a neuroanatomical perspective. Neuroscience. 2012;204:134–144. 10.1016/j.neuroscience.2011.07.052 [DOI] [PubMed] [Google Scholar]
- 53.Häring M, Guggenhuber S, Lutz B. Neuronal populations mediating the effects of endocannabinoids on stress and emotionality. Neuroscience. 2012;204:145–158. 10.1016/j.neuroscience.2011.12.035 [DOI] [PubMed] [Google Scholar]
- 54.Sadri-Vakili G, Bouzou B, Benn CL, Kim MO, Chawla P, Overland RP, et al. Histones associated with downregulated genes are hypo-acetylated in Huntington’s disease models. Hum Mol Genet. 2007;16:1293–1306. 10.1093/hmg/ddm078 [DOI] [PubMed] [Google Scholar]
- 55.Subbanna S, Nagre NN, Umapathy NS, Pace BS, Basavarajappa BS. Ethanol exposure induces neonatal neurodegeneration by enhancing CB1R Exon1 histone H4K8 acetylation and up-regulating CB1R function causing neurobehavioral abnormalities in adult mice. Int J Neuropsychopharmacol. 2014;18pii:pyu028. 10.1093/ijnp/pyu028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Prini P, Zamberletti E, Speziali S, Gabaglio M, Rainero A, Parolaro D, et al. Cannabinoids and gene expression: Adolescent THC exposure impacts genes involved in brain remodeling in the rat prefrontal cortex. Torino: Abstract for 36° Congresso Nazionale della Società Italiana di Farmacologia; 2013. Available: http://congresso.sifweb.org/archivio/cong36/abs/146.pdf. Accessed 28 March 2016.
- 57.Gangarossa G, Di Benedetto M, O’Sullivan GJ, Dunleavy M, Alcacer C, Bonito-Oliva A, et al. Convulsant doses of a dopamine D1 receptor agonist result in Erk-dependent increases in Zif268 and Arc/Arg3.1 expression in mouse dentate gyrus. PLoS One. 2011;6:e19415 10.1371/journal.pone.0019415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.González-Aparicio R, Moratalla R. Oleoylethanolamide reduces L-DOPA-induced dyskinesia via TRPV1 receptor in a mouse model of Parkinson’s disease. Neurobiol Dis. 2014;62:416–425. 10.1016/j.nbd.2013.10.008 [DOI] [PubMed] [Google Scholar]
- 59.Suri D, Bhattacharya A, Vaidya VA. Early stress evokes temporally distinct consequences on the hippocampal transcriptome, anxiety and cognitive behaviour. Int J Neuropsychopharmacol. 2014;17:289–301. 10.1017/S1461145713001004 [DOI] [PubMed] [Google Scholar]
- 60.Han A, Sung YB, Chung SY, Kwon MS. Possible additional antidepressant-like mechanism of sodium butyrate: targeting the hippocampus. Neuropharmacology. 2014;81:292–302. 10.1016/j.neuropharm.2014.02.017 [DOI] [PubMed] [Google Scholar]
- 61.Kyoto University Animal Experimentation Committee. Regulation on Animal Experimentation at Kyoto University. 2007. Available: http://www.kyoto-u.ac.jp/static/ja/research/ethic/arcku/2013/documents/03.pdf. Accessed 25 June 2015.
- 62.Armario A, Gil M, Marti J, Pol O, Balasch J. Influence of various acute stressors on the activity of adult male rats in a holeboard and in the forced swim test. Pharmacol Biochem Behav. 1991; 39:373–377. 10.1016/0091-3057(91)90194-7 [DOI] [PubMed] [Google Scholar]
- 63.Hayase T. Depression-related anhedonic behaviors caused by immobilization stress: a comparison with nicotine-induced depression-like behavioral alterations and effects of nicotine and/or “antidepressant” drugs. J Toxicol Sci. 2011;36:31–41. 10.2131/jts.36.31 [DOI] [PubMed] [Google Scholar]
- 64.Yoshida T, Sakane N, Umekawa T, Kondo M. Effect of nicotine on sympathetic nervous system activity of mice subjected to immobilization stress. Physiol Behav. 1994;55:53–57. 10.1016/0031-9384(94)90009-4 [DOI] [PubMed] [Google Scholar]
- 65.de Angelis L. Effects of valproate and lorazepam on experimental anxiety: tolerance, withdrawal, and role of clonidine. Pharmacol Biochem Behav. 1995;52:329–333. 10.1016/0091-3057(95)00100-B [DOI] [PubMed] [Google Scholar]
- 66.Takahashi RN, Pamplona FA, Fernandes MS. The cannabinoid antagonist SR141716A facilitates memory acquisition and consolidation in the mouse elevated T-maze. Neurosci Lett. 2005;380:270–275. 10.1016/j.neulet.2005.01.049 [DOI] [PubMed] [Google Scholar]
- 67.Li S, Murakami Y, Wang M, Maeda K, Matsumoto K. The effects of chronic valproate and diazepam in a mouse model of posttraumatic stress disorder. Pharmacol Biochem Behav. 2006;85:324–331. 10.1016/j.pbb.2006.08.015 [DOI] [PubMed] [Google Scholar]
- 68.McMahon LR, Koek W. Differences in the relative potency of SR 141716A and AM 251 as antagonists of various in vivo effects of cannabinoid agonists in C57BL/6J mice. Eur J Pharmacol. 2007;569:70–76. 10.1016/j.ejphar.2007.04.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gundersen BB, Blendy JA. Effects of the histone deacetylase inhibitor sodium butyrate in models of depression and anxiety. Neuropharmacology. 2009;57:67–74. 10.1016/j.neuropharm.2009.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chegini HR, Nasehi M, Zarrindast MR. Differential role of the basolateral amygdala 5-HT3 and 5-HT4 serotonin receptors upon ACPA-induced anxiolytic-like behaviors and emotional memory deficit in mice. Behav Brain Res. 2014;261:114–126. 10.1016/j.bbr.2013.12.007 [DOI] [PubMed] [Google Scholar]
- 71.Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–167. 10.1016/0165-0270(85)90031-7 [DOI] [PubMed] [Google Scholar]
- 72.File SE, Aranko K. Sodium valproate and chlordiazepoxide in the elevated plus-maze test of anxiety in the rat. Neuropsychobiology. 1988;20:82–86. 10.1159/000118478 [DOI] [PubMed] [Google Scholar]
- 73.Prior H, Schwegler H, Marashi V, Sachser N. Exploration, emotionality, and hippocampal mossy fibers in nonaggressive AB/Gat and congenic highly aggressive mice. Hippocampus. 2004;14:135–140. 10.1002/hipo.10166 [DOI] [PubMed] [Google Scholar]
- 74.Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. 10.1038/266730a0 [DOI] [PubMed] [Google Scholar]
- 75.Hayase T, Yamamoto Y, Yamamoto K. Stress-related behavioral alterations accompanying cocaine toxicity: the effects of mixed opioid drugs. Nihon Arukoru Yakubutsu Igakkai Zasshi. 2000;35:402–414. [PubMed] [Google Scholar]
- 76.Alves SH, Pinheiro G, Motta V, Landeira-Fernandez J, Cruz AP. Anxiogenic effects in the rat elevated plus-maze of 5-HT(2C) agonists into ventral but not dorsal hippocampus. Behav Pharmacol. 2004;15:37–43. 10.1097/01.fbp.0000113327.49506.87 [DOI] [PubMed] [Google Scholar]
- 77.Hayase T. Differential effects of TRPV1 receptor ligands against nicotine-induced depression-like behaviors. BMC Pharmacol. 2011;11:6 10.1186/1471-2210-11-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Biala G, Budzynska B. Effects of acute and chronic nicotine on elevated plus maze in mice: involvement of calcium channels. Life Sci. 2006;79:81–88. 10.1016/j.lfs.2005.12.043 [DOI] [PubMed] [Google Scholar]
- 79.Xing B, Liu P, Jiang WH, Liu F, Zhang H, Cao GF, et al. Effects of immobilization stress on emotional behaviors in dopamine D3 receptor knockout mice. Behav Brain Res. 2013;243:261–266. 10.1016/j.bbr.2013.01.019 [DOI] [PubMed] [Google Scholar]
- 80.Fisher DJ, Daniels R, Jaworska N, Knobelsdorf A, Knott VJ. Effects of acute nicotine administration on behavioral and neural (EEG) correlates of working memory in non-smokers. Brain Res. 2012;1429:72–81. 10.1016/j.brainres.2011.10.029 [DOI] [PubMed] [Google Scholar]
- 81.Grundey J, Thirugnanasambandam N, Kaminsky K, Drees A, Skwirba AC, Lang N, et al. Rapid effect of nicotine intake on neuroplasticity in non-smoking humans. Front Pharmacol. 2012;3:186 10.3389/fphar.2012.00186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Grundey J, Amu R, Ambrus GG, Batsikadze G, Paulus W, Nitsche MA. Double dissociation of working memory and attentional processes in smokers and non-smokers with and without nicotine. Psychopharmacology (Berl). 2015;232:2491–2501. 10.1007/s00213-015-3880-7 [DOI] [PubMed] [Google Scholar]
- 83.Dunlop BW, Nemeroff CB. The role of dopamine in the pathophysiology of depression. Arch Gen Psychiatry. 2007;64:327–337. 10.1001/archpsyc.64.3.327 [DOI] [PubMed] [Google Scholar]
- 84.Nikolaus S, Antke C, Müller HW. In vivo imaging of synaptic function in the central nervous system: II. Mental and affective disorders. Behav Brain Res. 2009;204:32–66. 10.1016/j.bbr.2009.06.009 [DOI] [PubMed] [Google Scholar]
- 85.Nikolaus S, Antke C, Beu M, Müller HW. Cortical GABA, striatal dopamine and midbrain serotonin as the key players in compulsive and anxiety disorders—results from in vivo imaging studies. Rev Neurosci. 2010;21:119–139. 10.1515/REVNEURO.2010.21.2.119 [DOI] [PubMed] [Google Scholar]
- 86.Hale MW, Shekhar A, Lowry CA. Stress-related serotonergic systems: implications for symptomatology of anxiety and affective disorders. Cell Mol Neurobiol. 2012;32:695–708. 10.1007/s10571-012-9827-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.File SE. Recent developments in anxiety, stress, and depression. Pharmacol Biochem Behav. 1996;54: 3–12. 10.1016/0091-3057(95)02175-2 [DOI] [PubMed] [Google Scholar]
- 88.Philip NS, Carpenter LL, Tyrka AR, Price LH. Nicotinic acetylcholine receptors and depression: a review of the preclinical and clinical literature. Psychopharmacology (Berl). 2010;212:1–12. 10.1007/s00213-010-1932-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen YW, Rada PV, Bützler BP, Leibowitz SF, Hoebel BG. Corticotropin-releasing factor in the nucleus accumbens shell induces swim depression, anxiety, and anhedonia along with changes in local dopamine/acetylcholine balance. Neuroscience. 2012;206:155–166. 10.1016/j.neuroscience.2011.12.009 [DOI] [PubMed] [Google Scholar]
- 90.File SE, Kenny PJ, Cheeta S. The role of the dorsal hippocampal serotonergic and cholinergic systems in the modulation of anxiety. Pharmacol Biochem Behav. 2000;66:65–72. 10.1016/S0091-3057(00)00198-2 [DOI] [PubMed] [Google Scholar]
- 91.Mineur YS, Obayemi A, Wigestrand MB, Fote GM, Calarco CA, Li AM, et al. Cholinergic signaling in the hippocampus regulates social stress resilience and anxiety- and depression-like behavior. Proc Natl Acad Sci U S A. 2013;110:3573–3578. 10.1073/pnas.1219731110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Newman MB, Nazian SJ, Sanberg PR. Diamond DM, Shytle RD. Corticosterone-attenuating and anxiolytic properties of mecamylamine in the rat. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:609–620. 10.1016/s0278-5846(00)00178-0 [DOI] [PubMed] [Google Scholar]
- 93.Spencer SJ, Ebner K, Day TA. Differential involvement of rat medial prefrontal cortex dopamine receptors in modulation of hypothalamic-pituitary-adrenal axis responses to different stressors. Eur J Neurosci. 2004;20:1008–1016. 10.1111/j.1460-9568.2004.03569.x [DOI] [PubMed] [Google Scholar]
- 94.Harrist A, Beech RD, King SL, Zanardi A, Cleary MA, Caldarone BJ, et al. Alteration of hippocampal cell proliferation in mice lacking the beta 2 subunit of the neuronal nicotinic acetylcholine receptor. Synapse. 2004;54:200–206. 10.1002/syn.20081 [DOI] [PubMed] [Google Scholar]
- 95.Morilak DA, Barrera G, Echevarria DJ, Garcia AS, Hernandez A, Ma S, et al. Role of brain norepinephrine in the behavioral response to stress. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1214–1224. 10.1016/j.pnpbp.2005.08.007 [DOI] [PubMed] [Google Scholar]
- 96.Stone EA, Lehmann ML, Lin Y, Quartermain D. Reduced evoked fos expression in activity-related brain regions in animal models of behavioral depression. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1196–1207. 10.1016/j.pnpbp.2007.04.010 [DOI] [PubMed] [Google Scholar]
- 97.Lanfumey L, Mongeau R, Cohen-Salmon C, Hamon M. Corticosteroid-serotonin interactions in the neurobiological mechanisms of stress-related disorders. Neurosci Biobehav Rev. 2008;32:1174–1184. 10.1016/j.neubiorev.2008.04.006 [DOI] [PubMed] [Google Scholar]
- 98.Molteni R, Calabrese F, Chourbaji S, Brandwein C, Racagni G, Gass P, et al. Depression-prone mice with reduced glucocorticoid receptor expression display an altered stress-dependent regulation of brain-derived neurotrophic factor and activity-regulated cytoskeleton-associated protein. J Psychopharmacol. 2010;24:595–603. 10.1177/0269881108099815 [DOI] [PubMed] [Google Scholar]
- 99.Benekareddy M, Vadodaria KC, Nair AR, Vaidya VA. Postnatal serotonin type 2 receptor blockade prevents the emergence of anxiety behavior, dysregulated stress-induced immediate early gene responses, and specific transcriptional changes that arise following early life stress. Biol Psychiatry. 2011;70:1024–1032. 10.1016/j.biopsych.2011.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schmuckermair C, Gaburro S, Sah A, Landgraf R, Sartori SB, Singewald N. Behavioral and neurobiological effects of deep brain stimulation in a mouse model of high anxiety- and depression-like behavior. Neuropsychopharmacology. 2013;38:1234–1244. 10.1038/npp.2013.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Takamura N, Nakagawa S, Masuda T, Boku S, Kato A, Song N, et al. The effect of dopamine on adult hippocampal neurogenesis. Prog Neuropsychopharmacol Biol Psychiatry. 2014;50:116–124. 10.1016/j.pnpbp.2013.12.011 [DOI] [PubMed] [Google Scholar]
- 102.Kinlein SA, Wilson CD, Karatsoreos IN. Dysregulated hypothalamic-pituitary-adrenal axis function contributes to altered endocrine and neurobehavioral responses to acute stress. Front Psychiatry. 2015;6:31 10.3389/fpsyt.2015.00031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pathak G, Ibrahim BA, McCarthy SA, Baker K, Kelly MP. Amphetamine sensitization in mice is sufficient to produce both manic- and depressive-related behaviors as well as changes in the functional connectivity of corticolimbic structures. Neuropharmacology. 2015;95:434–447. 10.1016/j.neuropharm.2015.04.026 [DOI] [PubMed] [Google Scholar]
- 104.Mineur YS, Fote GM, Blakeman S, Cahuzac EL, Newbold SA, Picciotto MR. Multiple nicotinic acetylcholine receptor subtypes in the mouse amygdala regulate affective behaviors and response to social stress. Neuropsychopharmacology. 2016;41:1579–1587. 10.1038/npp.2015.316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Morse DE. Neuroendocrine responses to nicotine and stress: enhancement of peripheral stress responses by the administration of nicotine. Psychopharmacology (Berl). 1989;98:539–543. 10.1007/BF00441956 [DOI] [PubMed] [Google Scholar]
- 106.Shingo AS, Kito S. Effects of nicotine on neurogenesis and plasticity of hippocampal neurons. J Neural Transm (Vienna). 2005;112:1475–1478. 10.1007/s00702-005-0370-2 [DOI] [PubMed] [Google Scholar]
- 107.Loughlin SE, Islas MI, Cheng MY, Lee AG, Villegier AS, Leslie FM. Nicotine modulation of stress-related peptide neurons. J Comp Neurol. 2006;497:575–588. 10.1002/cne.20999 [DOI] [PubMed] [Google Scholar]
- 108.Lutfy K, Brown MC, Nerio N, Aimiuwu O, Tran B, Anghel A, et al. Repeated stress alters the ability of nicotine to activate the hypothalamic-pituitary-adrenal axis. J Neurochem. 2006;99:1321–1327. 10.1111/j.1471-4159.2006.04217.x [DOI] [PubMed] [Google Scholar]
- 109.Schiltz CA, Kelley AE, Landry CF. Acute stress and nicotine cues interact to unveil locomotor arousal and activity-dependent gene expression in the prefrontal cortex. Biol Psychiatry. 2007;61:127–135. 10.1016/j.biopsych.2006.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gilbert DG, Meliska CJ, Plath LC. Noise stress does not modulate effects of smoking/nicotine on beta-endorphin, cortisol, ACTH, glucose, and mood. Psychopharmacology (Berl). 1997;130:197–202. 10.1007/s002130050229 [DOI] [PubMed] [Google Scholar]
- 111.Faraday MM, O’Donoghue VA, Grunberg NE. Effects of nicotine and stress on startle amplitude and sensory gating depend on rat strain and sex. Pharmacol Biochem Behav. 1999;62:273–284. 10.1016/S0091-3057(98)00159-2 [DOI] [PubMed] [Google Scholar]
- 112.Faraday MM, O’Donoghue VA, Grunberg NE. Effects of nicotine and stress on locomotion in Sprague-Dawley and Long-Evans male and female rats. Pharmacol Biochem Behav. 2003;74:325–333. 10.1016/S0091-3057(02)00999-1 [DOI] [PubMed] [Google Scholar]
- 113.Kaplan GB, Moore KA. The use of cognitive enhancers in animal models of fear extinction. Pharmacol Biochem Behav. 2011;99:217–228. 10.1016/j.pbb.2011.01.009 [DOI] [PubMed] [Google Scholar]
- 114.Kobayashi H, Iwata M, Mitani H, Yamada T, Nakagome K, Kaneko K.Valproic acid improves the tolerance for the stress in learned helplessness rats. Neurosci Res. 2012;72:355–363. 10.1016/j.neures.2011.12.009 [DOI] [PubMed] [Google Scholar]
- 115.Ookubo M, Kanai H, Aoki H, Yamada N. Antidepressants and mood stabilizers effects on histone deacetylase expression in C57BL/6 mice: Brain region specific changes. J Psychiatr Res. 2013;47:1204–1214. 10.1016/j.jpsychires.2013.05.028 [DOI] [PubMed] [Google Scholar]
- 116.Liu D, Qiu HM, Fei HZ, Hu XY, Xia HJ, Wang LJ, et al. Histone acetylation and expression of mono-aminergic transmitters synthetases involved in CUS-induced depressive rats. Exp Biol Med (Maywood). 2014;239:330–336. 10.1177/1535370213513987 [DOI] [PubMed] [Google Scholar]
- 117.Sailaja BS, Cohen-Carmon D, Zimmerman G, Soreq H, Meshorer E. Stress-induced epigenetic transcriptional memory of acetylcholinesterase by HDAC4. Proc Natl Acad Sci U S A. 2012;109:E3687–3695. 10.1073/pnas.1209990110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gozen O, Balkan B, Yildirim E, Koylu EO, Pogun S. The epigenetic effect of nicotine on dopamine D1 receptor expression in rat prefrontal cortex. Synapse. 2013;67:545–552. 10.1002/syn.21659 [DOI] [PubMed] [Google Scholar]
- 119.Umka J, Mustafa S, ElBeltagy M, Thorpe A, Latif L, Bennett G, et al. Valproic acid reduces spatial working memory and cell proliferation in the hippocampus. Neuroscience. 2010;166:15–22. 10.1016/j.neuroscience.2009.11.073 [DOI] [PubMed] [Google Scholar]
- 120.Papadopoulos A, Chandramohan Y, Collins A, Droste SK, Nutt DJ, Reul JM. GABAergic control of novelty stress-responsive epigenetic and gene expression mechanisms in the rat dentate gyrus. Eur Neuropsychopharmacol. 2011;21:316–324. 10.1016/j.euroneuro.2010.05.005 [DOI] [PubMed] [Google Scholar]
- 121.Dyrvig M, Hansen HH, Christiansen SH, Woldbye DP, Mikkelsen JD, Lichota J. Epigenetic regulation of Arc and c-Fos in the hippocampus after acute electroconvulsive stimulation in the rat. Brain Res Bull. 2012;88:507–513. 10.1016/j.brainresbull.2012.05.004 [DOI] [PubMed] [Google Scholar]
- 122.Moonat S, Sakharkar AJ, Zhang H, Tang L, Pandey SC. Aberrant histone deacetylase2-mediated histone modifications and synaptic plasticity in the amygdala predisposes to anxiety and alcoholism. Biol Psychiatry. 2013;73:763–773. 10.1016/j.biopsych.2013.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gagliano H, Delgado-Morales R, Sanz-Garcia A, Armario A. High doses of the histone deacetylase inhibitor sodium butyrate trigger a stress-like response. Neuropharmacology. 2014;79:75–82. 10.1016/j.neuropharm.2013.10.031 [DOI] [PubMed] [Google Scholar]
- 124.Ferland CL, Harris EP, Lam M, Schrader LA. Facilitation of the HPA axis to a novel acute stress following chronic stress exposure modulates histone acetylation and the ERK/MAPK pathway in the dentate gyrus of male rats. Endocrinology. 2014;155:2942–2952. 10.1210/en.2013-1918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ji S, Tian Y, Lu Y, Sun R, Ji J, Zhang L, et al. Irradiation-induced hippocampal neurogenesis impairment is associated with epigenetic regulation of bdnf gene transcription. Brain Res. 2014;1577:77–88. 10.1016/j.brainres.2014.06.035 [DOI] [PubMed] [Google Scholar]
- 126.Petraglia F, Bakalakis S, Facchinetti F, Volpe A, Muller EE, Genazzani AR. Effects of sodium valproate and diazepam on beta-endorphin, beta-lipotropin and cortisol secretion induced by hypoglycemic stress in humans. Neuroendocrinology. 1986;44:320–325. 10.1159/000124663 [DOI] [PubMed] [Google Scholar]
- 127.Biala G, Kruk M, Budzynska B. Effects of the cannabinoid receptor ligands on anxiety-related effects of d-amphetamine and nicotine in the mouse elevated plus maze test. J Physiol Pharmacol. 2009;60:113–122. [PubMed] [Google Scholar]
- 128.Fokos S, Panagis G. Effects of delta9-tetrahydrocannabinol on reward and anxiety in rats exposed to chronic unpredictable stress. J Psychopharmacol. 2010;24:767–777. 10.1177/0269881109104904 [DOI] [PubMed] [Google Scholar]
- 129.Bambico FR, Hattan PR, Garant JP, Gobbi G. Effect of delta-9-tetrahydrocannabinol on behavioral despair and on pre- and postsynaptic serotonergic transmission. Prog Neuropsychopharmacol Biol Psychiatry. 2012;38:88–96. 10.1016/j.pnpbp.2012.02.006 [DOI] [PubMed] [Google Scholar]
- 130.Häring M, Grieb M, Monory K, Lutz B, Moreira FA. Cannabinoid CB1 receptor in the modulation of stress coping behavior in mice: the role of serotonin and different forebrain neuronal subpopulations. Neuropharmacology. 2013;65:83–89. 10.1016/j.neuropharm.2012.09.002 [DOI] [PubMed] [Google Scholar]
- 131.Häring M, Marsicano G, Lutz B, Monory K. Identification of the cannabinoid receptor type 1 in serotonergic cells of raphe nuclei in mice. Neuroscience. 2007;146:1212–1219. 10.1016/j.neuroscience.2007.02.021 [DOI] [PubMed] [Google Scholar]
- 132.Patel S, Roelke CT, Rademacher DJ, Cullinan WE, Hillard CJ. Endocannabinoid signaling negatively modulates stress-induced activation of the hypothalamic-pituitary-adrenal axis. Endocrinology. 2004;145:5431–5438. 10.1210/en.2004-0638 [DOI] [PubMed] [Google Scholar]
- 133.Jiang W, Zhang Y, Xiao L, Van Cleemput J, Ji SP, Bai G, et al. Cannabinoids promote embryonic and adult hippocampus neurogenesis and produce anxiolytic- and antidepressant-like effects. J Clin Invest. 2005;115:3104–3116. 10.1172/JCI25509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hill MN, Ho WS, Sinopoli KJ, Viau V, Hillard CJ, Gorzalka BB. Involvement of the endocannabinoid system in the ability of long-term tricyclic antidepressant treatment to suppress stress-induced activation of the hypothalamic-pituitary-adrenal axis. Neuropsychopharmacology. 2006;31:2591–2599. 10.1038/sj.npp.1301092 [DOI] [PubMed] [Google Scholar]
- 135.Rubino T, Sala M, Viganò D, Braida D, Castiglioni C, Limonta V, et al. Cellular mechanisms underlying the anxiolytic effect of low doses of peripheral Delta9-tetrahydrocannabinol in rats. Neuropsychopharmacology. 2007;32:2036–2045. 10.1038/sj.npp.1301330 [DOI] [PubMed] [Google Scholar]
- 136.Braida D, Limonta V, Malabarba L, Zani A, Sala M. 5-HT1A receptors are involved in the anxiolytic effect of Delta9-tetrahydrocannabinol and AM 404, the anandamide transport inhibitor, in Sprague-Dawley rats. Eur J Pharmacol. 2007;555:156–163. 10.1016/j.ejphar.2006.10.038 [DOI] [PubMed] [Google Scholar]
- 137.Haller J, Mátyás F, Soproni K, Varga B, Barsy B, Németh B, et al. Correlated species differences in the effects of cannabinoid ligands on anxiety and on GABAergic and glutamatergic synaptic transmission. Eur J Neurosci. 2007;25:2445–2456. 10.1111/j.1460-9568.2007.05476.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hill MN, Gorzalka BB. Enhancement of anxiety-like responsiveness to the cannabinoid CB(1) receptor agonist HU-210 following chronic stress. Eur J Pharmacol. 2004;499:291–295. 10.1016/j.ejphar.2004.06.069 [DOI] [PubMed] [Google Scholar]
- 139.Griebel G, Stemmelin J, Scatton B. Effects of the cannabinoid CB1 receptor antagonist rimonabant in models of emotional reactivity in rodents. Biol Psychiatry. 2005;57:261–267. 10.1016/j.biopsych.2004.10.032 [DOI] [PubMed] [Google Scholar]
- 140.Steiner MA, Marsicano G, Nestler EJ, Holsboer F, Lutz B, Wotjak CT. Antidepressant-like behavioral effects of impaired cannabinoid receptor type 1 signaling coincide with exaggerated corticosterone secretion in mice. Psychoneuroendocrinology. 2008;33:54–67. 10.1016/j.psyneuen.2007.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Egashira N, Matsuda T, Koushi E, Higashihara F, Mishima K, Chidori S, et al. Delta(9)-tetrahydrocannabinol prolongs the immobility time in the mouse forced swim test: involvement of cannabinoid CB(1) receptor and serotonergic system. Eur J Pharmacol. 2008;589:117–121. 10.1016/j.ejphar.2008.03.046 [DOI] [PubMed] [Google Scholar]
- 142.Marco EM, Pérez-Alvarez L, Borcel E, Rubio M, Guaza C, Ambrosio E, et al. Involvement of 5-HT1A receptors in behavioural effects of the cannabinoid receptor agonist CP 55,940 in male rats. Behav Pharmacol. 2004;15:21–27. 10.1097/00008877-200402000-00003 [DOI] [PubMed] [Google Scholar]
- 143.Rey AA, Purrio M, Viveros MP, Lutz B. Biphasic effects of cannabinoids in anxiety responses: CB1 and GABA(B) receptors in the balance of GABAergic and glutamatergic neurotransmission. Neuropsychopharmacology. 2012;37:2624–2634. 10.1038/npp.2012.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Takahashi E, Katayama M, Niimi K, Itakura C. Additive subthreshold dose effects of cannabinoid CB(1) receptor antagonist and selective serotonin reuptake inhibitor in antidepressant behavioral tests. Eur J Pharmacol. 2008;589:149–156. 10.1016/j.ejphar.2008.05.020 [DOI] [PubMed] [Google Scholar]
- 145.Kelsey JE, Harris O, Cassin J. The CB(1) antagonist rimonabant is adjunctively therapeutic as well as monotherapeutic in an animal model of Parkinson's disease. Behav Brain Res. 2009;203:304–307. 10.1016/j.bbr.2009.04.035 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.