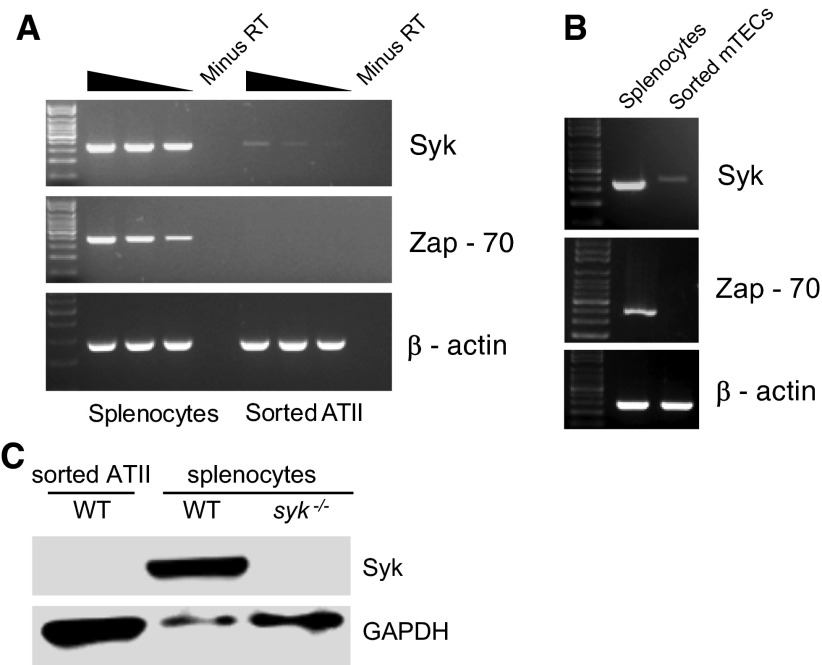
Figure 3.
Expression of Syk family kinases in sorted ATII cells and mTECs. RNA isolated from highly pure, flow-sorted ATII cells (A) and mTECs (B) was analyzed by RT-PCR, as described in Materials and Methods, to assess expression of Syk and Zap-70. RNA prepared from total splenocytes was used as a positive control. For analysis of ATII cells, undiluted, threefold, and ninefold dilutions of cDNA from ATII cells or positive control were used for each lane, as indicated by the gradient bar. For each PCR, a no–reverse transcriptase (minus RT) control was run alongside to demonstrate that the signal was not due to genomic DNA contamination. For analysis of mTECs, only a single cDNA concentration was tested, as RNA was limiting. As loading control, RT-PCR for β-actin was performed. Results shown are representative of three independent experiments. (C) Western blotting for Syk, with glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as loading control, was performed using sorted wild-type (WT) ATII cells, WT splenocytes, and syk−/− splenocytes harvested from spleens of syk−/− chimeric mice. WT ATII cell lysate was purposely overloaded to look for low-level Syk protein expression.