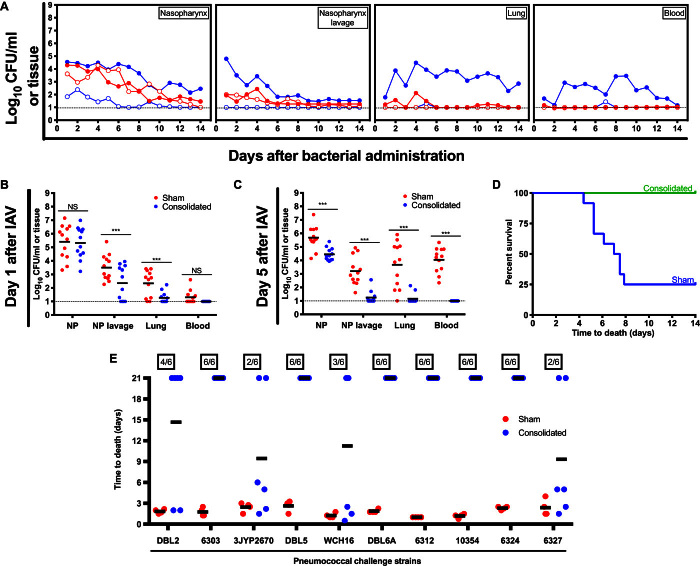
Fig. 4. Directed and extended protection using the hybrid vector.
(A) Assessment of bacterial burden was conducted across anatomical sites for unimmunized (filled circles) and immunized (using the consolidated antigens; open circles) mice challenged with avirulent (planktonic; red) or virulent (biofilm-released; blue) S. pneumoniae strain EF3030. (B to D) Site-specific bacterial burden and protection were also tested over time for mice colonized with the S. pneumoniae strain EF3030 and triggered for virulence progression using influenza A virus (IAV) inoculation. Dotted lines represent limit of detection for bacterial counts. ***P < 0.001. (E) Protection assessment (using a mouse sepsis challenge model) was then extended to 10 additional clinically relevant S. pneumoniae strains.