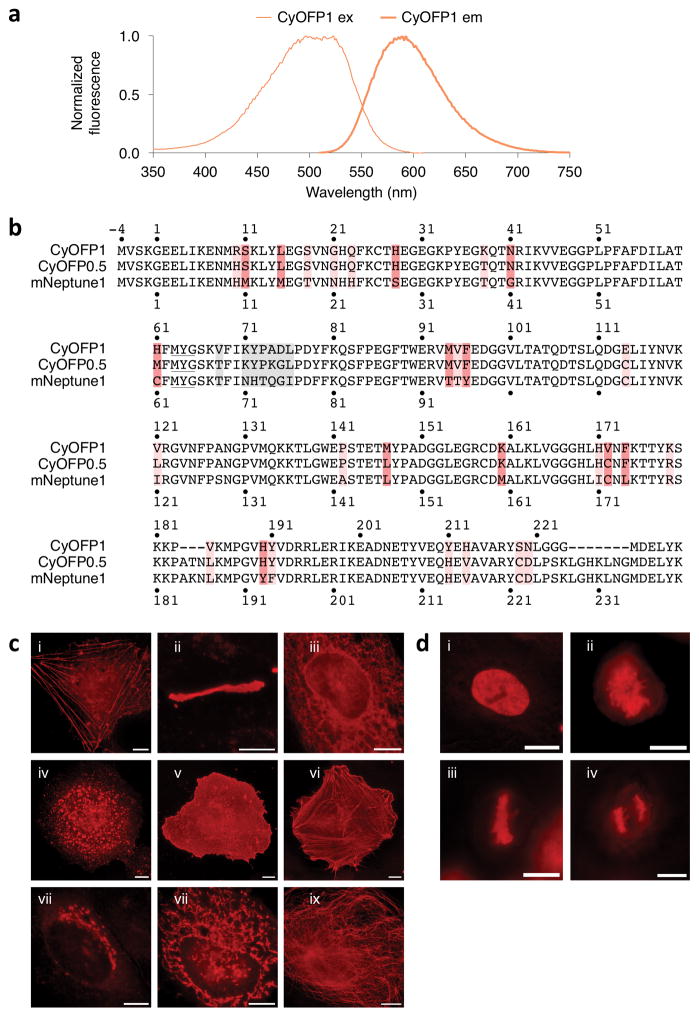
Figure 1.
Development of CyOFP1, a cyan-excitable red fluorescent protein. (a) Normalized excitation and emission spectra of CyOFP1. (b) Sequence alignment of CyOFP1 and mNeptune1. Amino acids forming the chromophore are underlined. Interior mutations are in red and outer barrel mutations are colored pink. Mutations in the upper loop (when the barrel is oriented with termini pointing upwards) are colored gray. Amino acid numbering begins at −4 so that homologous sequences are numbered as in PDB file 3IP2 for the structure of Neptune. (c) Fluorescence images of HeLa CCL2 cells expressing CyOFP1 fused to various domains. For each fusion, the linker amino acid (aa) length is indicated in between the two domains, and the origin of the fusion partner and its normal subcellular location are indicated in parentheses. i, CyOFP1-18aa-actin (β-actin, actin cytoskeleton); ii, Cx43-7aa-CyOFP1 (rat α-1 connexin 43, gap junctions); iii, CytERM-17aa-CyOFP1 (rabbit cytochrome p450 aa1-29, endoplasmic reticulum); iv, CyOFP1-14aa-RhoB (human RhoB, endosomes); v, CyOFP1-5aa-CAAX (human c-Ha-Ras 20- aa farnesylation signal, plasma membrane); vi, Tractin-11aa-CyOFP1 (rat F-Tractin; actin cytoskeleton); vii, SiT-7aa-CyOFP1, human sialyltransferase aa1-45, Golgi apparatus); viii, COX8A-7aa-CyOFP1 (human cytochrome C oxidase subunit VIIIA; mitochondria); ix, CyOFP1-18aa-tubulin (human α-tubulin, microtubules). (d) Fluorescence images of CyOFP1-10aa-H2B (human histone 2B) in HeLa S3 cells; i, interphase; ii, prophase; iii, metaphase; iv, anaphase. Scale bars, 10 Mm.