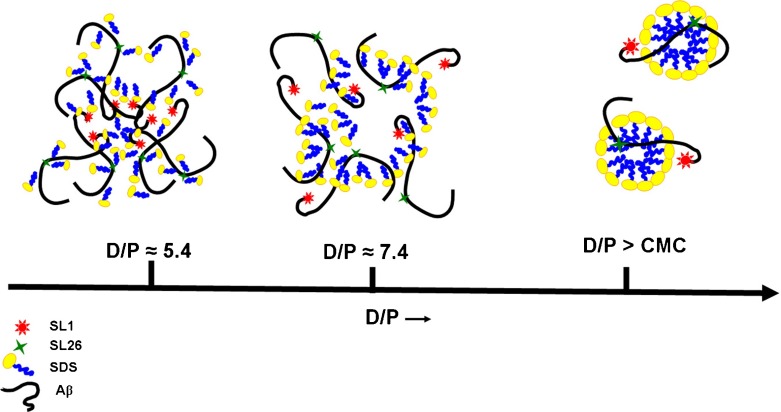
Fig. 6.
Illustration of the A β aggregation at different D/P ratios. On the left side, the A β aggregate is shown at a D/P of about 5.4, in which the hydrophilic N-terminus becomes immobilized at the aggregate–buffer interface. This helps to solubilize the aggregate. In the middle, the A β aggregate is shown at D/P ratios of about 7.3, where there are sufficient SDS molecules to replace (some of) the A β N-termini at the water–aggregate interface. On the right, the A β peptide is shown at D/P ratios above the CMC of SDS. Two possible models for A β interaction with a micelle are shown, in which both spin labels would have similar rotation correlation times