Abstract
Purpose
Sacubitril/valsartan (LCZ696) is a first-in-class angiotensin receptor neprilysin inhibitor (ARNI) indicated to reduce the risk of cardiovascular death and hospitalization for heart failure in patients with chronic heart failure (NYHA class II–IV) and reduced ejection fraction. This study was aimed to evaluate the effect of single oral therapeutic (400 mg) and supratherapeutic (1200 mg) doses of LCZ696 on cardiac repolarization.
Method
This randomized double-blind crossover study in healthy male subjects compared the effect of therapeutic and supratherapeutic doses of LCZ696 with placebo and moxifloxacin 400 mg (open-label treatment) as positive control. The primary assessment was mean baseline- and placebo-corrected QTcF (∆∆QTcF; Fridericia correction). Additional assessments included the ∆∆QTcB (Bazett’s correction), PR interval, QRS duration, heart rate (HR), LCZ696 pharmacokinetics, pharmacokinetic/pharmacodynamic relationships, and safety.
Results
Of the 84 subjects enrolled, 81 completed the study. The maximum upper bound of the two-sided 90 % confidence interval for ∆∆QTcF for LCZ696 400 mg and 1200 mg were <10 ms, and assay sensitivity was confirmed with moxifloxacin. No relevant treatment-emergent changes were observed in any of the ECG-derived parameters with LCZ696 or placebo, and the incidence of adverse events was comparable among the treatment groups.
Conclusion
Single therapeutic and supratherapeutic doses of LCZ696 did not affect cardiac repolarization as defined by the E14 ICH guidelines.
Electronic supplementary material
The online version of this article (doi:10.1007/s00228-016-2062-9) contains supplementary material, which is available to authorized users.
Keywords: Angiotensin receptor neprilysin inhibitor, Cardiac repolarization, Heart failure, LCZ696, QT prolongation, Sacubitril/valsartan
Introduction
Sacubitril/valsartan (LCZ696) is a first-in-class angiotensin receptor neprilysin inhibitor (ARNI) indicated to reduce the risk of cardiovascular death and hospitalization for heart failure in patients with chronic heart failure (NYHA class II–IV) and reduced ejection fraction (HFrEF) [1, 2]. Following oral administration, LCZ696 provides systemic exposure to the pro-drug sacubitril (also known as AHU377, which is further metabolized to the active neprilysin inhibitor LBQ657) and valsartan [3]. The mechanism of the beneficial effects of LCZ696 in HF are believed to be due to the enhancement of protective endogenous neurohormonal systems, such as the natriuretic peptide (NP) system, in the presence of simultaneous inhibition of the deleterious effects related to sustained renin-angiotensin-aldosterone system (RAAS) activation.
Drug-induced delayed cardiac repolarization, as reflected by a prolongation of the rate-corrected QT interval (QTc) is an important cardiac safety finding leading to the withdrawal of numerous drugs from the market [4, 5]. Drugs that have potential to block the rapid component of the delayed rectifier potassium channel (IKR) coded by human Ether-à-go-go related gene (hERG) can prolong QTc, which may lead to increased susceptibility to cardiac arrhythmias, notably Torsades de Pointes [4, 6, 7]. Therefore, rigorous characterization of novel pharmaceutical agents for their potential to prolong the QTc interval is warranted as part of their cardiac safety assessment.
Clinical and experimental evidence corroborate the beneficial effects of chronic treatment with RAAS inhibitors on the duration of QT interval in patients with hypertension and HF [8]. While this may reflect the end result of several indirect factors, the impact of RAAS inhibitors on the electrophysiological mechanisms altered by angiotensin II might be a possible reason [9]. Angiotensin II probably prolongs the action potential by inhibiting the rapidly activating components of IKR [10]. The beneficial effect of valsartan in HF is clinically evident, while sacubitril showed no relevant effect on the hERG/IKR channel current even at the highest LCZ696 concentration tested (3000 μM; <50 % inhibition) in vitro (data on file). Although in vitro ion-channel assessment of LBQ657 was not performed, QT prolongation was not documented during systematic in vivo exposure in primates (LCZ696) and dogs (sacubitril). This study therefore provided a dedicated assessment of effects of single oral therapeutic (400 mg) and supratherapeutic (1200 mg) doses of LCZ696 on cardiac repolarization to support the use in patients with HF.
Methods
Subjects
Healthy male subjects 18–45 years of age with a body weight ≥60 kg, a body mass index (BMI) of 18–28 kg/m2, and normal ECG, vital signs, and laboratory tests were enrolled in the study. The key exclusion criteria were use of other investigational drugs within 30 days of enrollment or within five half-lives of treatment, contraindications to the use of moxifloxacin, a history of angioedema or drug/alcohol abuse, or donation or loss of ≥400 mL of blood within 8 weeks before initial dosing. All subjects provided written informed consent before starting any of the study-related procedures.
Study design
This was a single-center, randomized, positive- and placebo-controlled, partially blinded, single-dose study with a 4 × 3 Williams crossover design with 12 treatment sequences (Fig. 1). Following a screening period of up to 21 days, eligible subjects entered the randomized double-blind treatment periods consisting of four 48-h treatment periods separated by washout periods of ≥4 days each. The final study completion evaluation was performed 4–10 days after the last treatment. Subjects were admitted at least 35 h before treatment in each treatment period for baseline assessments including 24-h ECG Holter recording and were permitted to leave the center during the washout periods.
Fig. 1.
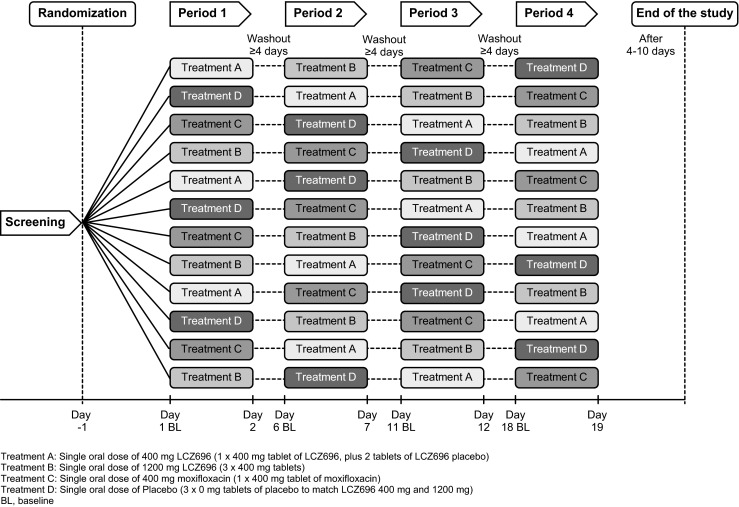
Study design. Treatment A: Single oral dose of 400 mg LCZ696 (1 × 400 mg tablet of LCZ696, plus 2 tablets of LCZ696 placebo). Treatment B: Single oral dose of 1200 mg LCZ696 (3 × 400 mg tablets). Treatment C: Single oral dose of 400 mg moxifloxacin (1 × 400 mg tablet of moxifloxacin). Treatment D: Single oral dose of placebo (3 × 0 mg tablets of placebo to match LCZ696 400 mg and 1200 mg). BL, baseline
The subjects were randomized to one of the 12 treatment sequences and received four treatments: single oral doses of LCZ696 400 mg (therapeutic dose), LCZ696 1200 mg (supratherapeutic dose), placebo, or moxifloxacin 400 mg. Treatments with LCZ696 and placebo were blinded, whereas moxifloxacin treatment was administered in an open-label manner.
Food and beverages that could potentially impact the PK of LCZ696 analytes or ECG measurements, such as citrus fruits, grapefruit, and quinine (e.g., tonic water), were restricted within 3 days before or during treatment periods, while alcohol and substances containing methylxanthines (e.g., caffeine, cola, chocolate) were restricted 10 h before the start of the continuous ECG measurement and during the clinic stay of each treatment period.
The study protocol was approved by the Ethics Committee of the study center. The study was designed and implemented in accordance with the ICH Guidelines for Good Clinical Practice E6 [11] and for Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs E14 [12], applicable local regulations, and the ethical principles laid down in the Declaration of Helsinki.
Pharmacodynamic assessments
The primary assessment was the mean baseline- and placebo-corrected Fridericia-corrected QT (∆∆QTcF). The secondary assessments included the ∆∆QTcB (Bazett’s correction); QTcF and QTcB assay sensitivity with moxifloxacin; proportion of subjects with notable changes in corrected (QTcF and QTcB) and uncorrected QT (defined as an increase of >30 or >60 ms from baseline or a change to >450, >480, or >500 ms, if the condition not existed at baseline); changes in PR interval, QRS duration, and HR and the proportion of subjects with notable changes from baseline in these parameters defined as 40 > HR > 100, PR > 200, and QRS > 110 ms in the presence of 25 % change from pretreatment.
Continuous 24-h Holter ECGs were collected at baseline and following drug administration in each treatment period. ECGs were extracted from the Holter recordings at −1 h, −35 min, and −15 min for baseline, and at 0.5, 1, 2, 3, 4, 5, 8, 12, and 24 h relative to the dosing time for post-dose assessment. ECGs were analyzed in a blinded fashion in a central ECG laboratory (iCardiac Technologies Inc., Rochester, NY, USA).
Pharmacokinetic assessments
The pharmacokinetic (PK) parameters including peak plasma drug concentration (Cmax), time to reach Cmax (Tmax), area under the plasma concentration-time curve from 0 to 24 h post-dose (AUC0–24 h), and AUC up to the last measurable concentration (AUClast) for sacubitril, LBQ657 and valsartan were determined by noncompartmental methods using WinNonlin Phoenix version 6.2 (Pharsight Corporation, Mountain View, CA). The exposure-response relationship for QTcF during LCZ696 treatment was also assessed.
For the assessments of PK parameters, serial blood samples were collected at predose and 0.5, 1, 2, 3, 4, 5, 8, 12, and 24 h post-dose. Plasma was obtained at each time point and kept frozen at ≤−15 °C until analysis. Quantification of sacubitril, LBQ657, and valsartan in plasma was performed using a validated liquid chromatography tandem mass spectroscopy (LC-MS/MS) method [3]. The lower limits of quantification for sacubitril, LBQ657, and valsartan were 1.00, 20.0, and 10.0 ng/mL, respectively.
Safety
Safety assessments included monitoring of all adverse events (AEs) and serious AEs (SAEs) and their relationship to study drugs. Vital signs and laboratory parameters were assessed at regularly intervals, and additional safety ECGs were collected and assessed to confirm subject safety during profile days.
Statistical analyses
A total of 84 subjects were planned to be randomized, assuming a dropout rate of <10 %, to complete all the four treatment periods of the study with at least 73 subjects. This would provide ≥80 % power to obtain the upper bound of the two-sided 90 % confidence interval (CI) for the comparison between LCZ696 and placebo below 10 ms, when the true difference between the two treatments was ≤4 ms, assuming a standard deviation (SD) of change from baseline in QTc of <11.5 ms.
The safety analysis set included all subjects receiving at least one dose of the study drug. The PD analysis set included all subjects with available PD data and no protocol deviations that could have an impact on the PD data. The PK analysis set included all subjects who received the study drug with at least one available valid PK concentration without protocol deviations that could have a relevant impact on the PK data.
For the assessment of the primary objective of the study, the effects of single therapeutic and supratherapeutic doses of LCZ696 on QTcF were compared with placebo using one-sided t tests at the level of α = 0.05 as per the requirements of the ICH E14 guidelines [12]. The null hypothesis under consideration was a change from baseline of placebo-corrected QTc (∆∆QTcF) >10 ms for at least one time point. The lack of effect on QTc was considered to be established upon the rejection of the null hypotheses, that is, if the upper bound of all two-sided 90 % confidence intervals (or one-sided 95 % upper bound) of ∆∆QTcF values at all the time points were below 10 ms for LCZ696. Assay sensitivity was concluded if the p value for at least one of the 4 comparisons at the 1, 2, 3, and 4 h post-dose time points for moxifloxacin was <0.0125 (Bonferroni correction).
Goodness-of-fit (best fit) graphs plotted to determine the performance of the QT correction formulas, based on the predose QTc-RR relationship (Supplementary Fig. S1), suggest the Fridericia correction method (QTcF) as an appropriate method as the Bazett correction method (QTcB) tended to overcorrect at elevated HR. The PK-PD relationship was evaluated using a linear mixed-effect model.
Results
Subjects
Of the total 84 randomized subjects, 81 subjects completed the study, with treatment noncompliance (one subject), difficulty in swallowing the tablet (one subject), and withdrawal of consent (one subject) being the reasons for discontinuations. The mean age of the enrolled subjects was 32.8 years, all subjects were men and majority of the subjects were Caucasians (96.4 %), and mean (standard deviation) weight was 79.0 (8.81) kg and a BMI of 24.5 (2.27) kg/m2 (Supplementary Table 1).
Pharmacodynamic assessments
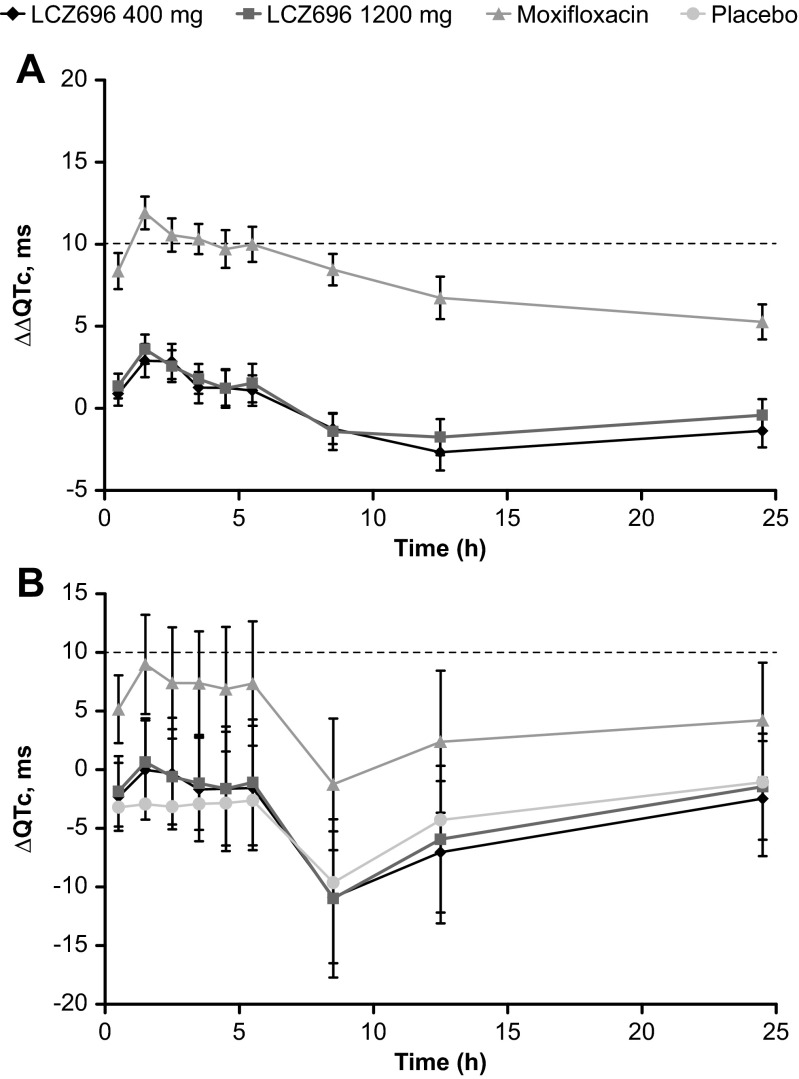
The maximum mean ∆∆QTcF following the administration of LCZ696 400 and 1200 mg was 2.9 and 3.6 ms, respectively, observed at 1 h post-dose. The upper bound of the two-sided 90 % CI for all the ∆∆QTcF values remained below the 10 ms threshold, with a maximum upper bound of 3.9 ms observed at 2 h post-dose for LCZ696 400 mg, and 4.5 ms observed at 1 h post-dose for LCZ696 1200 mg (Fig. 2a). The baseline-corrected QTcF (∆QTcF) comparing all treatments with placebo is presented in Fig. 2b.
Fig. 2.
QTcF following administration of LCZ696 400 mg, 1200 mg, moxifloxacin 400 mg, and placebo by time point: a Baseline- and placebo-corrected QTcF (∆∆QTcF) and b baseline-corrected QTcF (∆QTcF). Data are mean ± 95 % confidence intervals
Following moxifloxacin administration, the maximum mean ∆∆QTcF at 1 h post-dose was 11.9 ms, and the lower bounds of the two-sided 90 % CIs for ∆∆QTcF was >0 ms at all predefined post-dose time points, thereby confirming assay sensitivity.
The mean changes in HR, PR interval, and QRS duration from baseline by time and treatment are presented in Supplement Fig. S2a, b, c. The maximal mean HR increase from baseline was observed at 8 h after administration of LCZ696 400 mg (10.5 bpm) and LCZ696 1200 mg (11.4 bpm), and 12 h after administration of moxifloxacin and placebo (6.9 and 6.3 bpm, respectively). There were no treatment-related relevant changes in the mean PR interval and QRS duration in any of the treatment groups.
Notable changes in ECG parameters
The proportions of subjects with treatment-emergent notable changes in ECG parameters are presented in Supplementary Table 2. No subject had treatment-emergent QTcF or uncorrected QT values of >480 ms or >500 ms, or an increase from baseline by >60 ms. Treatment-emergent QTcF >450 ms occurred only in one subject receiving moxifloxacin from 1 to 5 h post-dose, while an uncorrected QT >450 ms was observed in 1 (1.2 %) subject receiving LCZ696 400 mg and in 3 (3.7 %) subjects receiving moxifloxacin. An increase in corrected QT from baseline by 30–60 ms was observed only in the moxifloxacin group (2 [2.5 %]). No treatment-emergent notable events related to changes in HR, PR interval, or QRS duration were noted during LCZ696 treatment. In addition, no changes in ECG related to repolarization or morphology were noted during LCZ696 treatment.
Pharmacokinetic assessments
Pharmacokinetics of sacubitril, LBQ657, and valsartan following the administration of single oral doses of LCZ696 400 or 1200 mg under fasting condition are summarized in Table 1. The mean plasma concentrations of sacubitril increased rapidly with a median Tmax of 0.52 h for the 400 mg dose and 1.05 h for the 1200 mg dose, followed by LBQ657, with the corresponding Tmax values of 2.07 and 3.05 h, respectively. The median Tmax for valsartan was 2.07 h for both the LCZ696 400 mg and 1200 mg doses. The Cmax of LBQ657 showed a dose proportional increase, while the Cmax of sacubitril and valsartan showed less than proportional increases between the doses. The arithmetic mean AUC0–24 h and AUClast for sacubitril and LBQ657 increased approximately dose proportionally, but showed less than dose proportional increase for valsartan.
Table 1.
Pharmacokinetics of LCZ696 analytes following administration of therapeutic (400 mg) and supratherapeutic doses (1200 mg) of LCZ696
| Parameter | LCZ696 400 mg | LCZ696 1200 mg | ||||
|---|---|---|---|---|---|---|
| N | Arithmetic mean (SD) | CV% | N | Arithmetic mean (SD) | CV% | |
| Sacubitril | ||||||
| AUC0–24 h, h*ng/mL | 81 | 4400 (1780) | 40.4 | 82 | 13200 (4640) | 35.3 |
| AUClast, h*ng/mL | 82 | 4390 (1760) | 40.2 | 82 | 13100 (4640) | 35.3 |
| Cmax, ng/mL | 82 | 3210 (1690) | 52.6 | 82 | 7780 (3830) | 49.2 |
| Tmax, ha | 82 | 0.52 (0.50, 3.08) | 82 | 1.05 (0.48, 4.0) | ||
| LBQ657 | ||||||
| AUC0–24 h, h*ng/mL | 81 | 122000 (19700) | 16.2 | 82 | 364000 (62300) | 17.1 |
| AUClast, h*ng/mL | 82 | 121000 (22200) | 18.3 | 82 | 364000 (62400) | 17.1 |
| Cmax, ng/mL | 82 | 13700 (2490) | 18.2 | 82 | 40700 (6990) | 17.2 |
| Tmax, ha | 82 | 2.07 (1.05, 5.07) | 82 | 3.05 (2.05, 5.07) | ||
| Valsartan | ||||||
| AUC0–24 h, h*ng/mL | 81 | 30500 (14500) | 47.5 | 82 | 66000 (25400) | 38.5 |
| AUClast, h*ng/mL | 82 | 30300 (14500) | 47.9 | 82 | 66000 (25400) | 38.5 |
| Cmax, ng/mL | 82 | 4690 (2210) | 47.2 | 82 | 9360 (3790) | 40.5 |
| Tmax, ha | 82 | 2.07 (1.05, 5.07) | 82 | 2.07 (1.03, 4.07) | ||
AUC 0–24 h area under the plasma concentration-time curve from 0 to 24 h post-dose, AUC last AUC up to the last measured concentration, C max peak plasma drug concentration, CV coefficient of variance, SD standard deviation, T max time to reach Cmax
aData are presented as median (range)
Pharmacokinetic and pharmacodynamics relationship
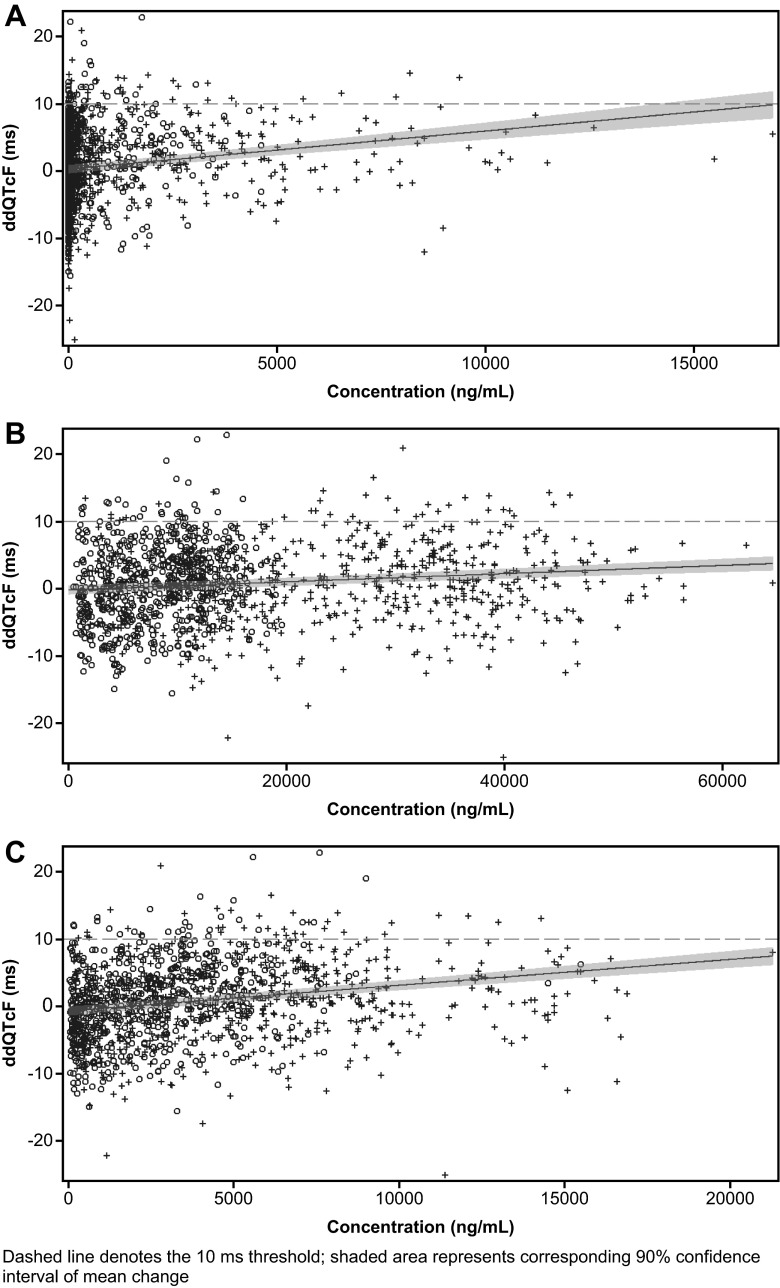
The estimated regression of plasma concentrations of LCZ696 analytes against ∆∆QTcF and the corresponding 90 % CI (shaded area) are presented in Fig. 3. The upper bounds of the model-predicted 90 % CI of the regression equation for ∆∆QTcF were below 10 ms for all LCZ696 analytes, except for sacubitril at concentrations >15000 ng/mL. The mean Cmax of sacubitril following administration of a single therapeutic dose of 400 mg LCZ696 was 3210 ng/mL.
Fig. 3.
Pharmacokinetic/pharmacodynamic relationship following LCZ696 administration for a sacubitril, b LBQ657, and c valsartan. Dashed line denotes the 10-ms threshold; shaded area represents corresponding 90% confidence interval of mean change
Safety
Of the 84 subjects enrolled and randomized, 29 subjects (34.5 %) experienced at least one AE during the study (Supplement Table 3). The overall incidence of AEs was comparable across the treatment groups (LCZ696 400 mg [14.6 %], LCZ696 1200 mg [14.6 %], moxifloxacin 400 mg [13.6 %], and placebo [12.2 %]). Headache, nasopharyngitis, postural dizziness, and nausea were the most commonly reported AEs. Majority of the AEs were of mild intensity and resolved without any treatment. No deaths or SAEs were reported during the study, and none of the AEs led to study discontinuation of a subject. There were no clinically significant changes in laboratory measurements, vital signs, and safety ECGs.
Discussion
Patients with heart failure have an impaired repolarization reserve [13], in addition to several other factors that may predispose them and contribute to potentially life-threatening cardiac arrhythmias, including autonomic imbalance, electrolyte abnormalities, and QT-prolonging co-treatments. They are also at significantly increased risk for malignant ventricular death and sudden death [14].
LCZ696 is a novel drug approved for the treatment of HFrEF. According to ICH E14 guidelines, a thorough assessment of its effects on cardiac repolarization is required. Whereas the autonomic and neurohormonal milieu present in patients with HF differs substantially from healthy subjects, the TQT study environment provides the optimally standardized environment required to identify modest underlying changes in cardiac repolarization, and studies in healthy subjects are generally considered acceptable to inform drug-related repolarization liability [12].
This study evaluated the effect of LCZ696 400 mg, representing the total daily dose of the highest approved clinical dose and regimen (200 mg twice daily), and 1200 mg, representing a 6-fold multitude of any given single dose and 3-fold multiple of the standard total daily dose. Plasma concentrations observed in the present QTc study exceeded observed steady-state plasma concentration in healthy subjects and patients with heart failure. Furthermore, all LCZ696 analytes have a short half-life, high-plasma protein binding and were fully characterized regarding their effect on the QTc interval, thereby justifying a single-dose study design and the selected doses.
The results indicate no relevant impact on cardiac repolarization with LCZ696 at either doses as evidenced by a mean ∆∆QTcF <5 ms with an upper bound <10 ms, in contrast to the positive comparator, moxifloxacin. LCZ696 administration in healthy subjects was associated with transient asymptomatic increase in HR. Changes in HR have the potential to impact the performance of rate-corrected QT values. Published literature indicates that the Fridericia formula performs better under conditions of alterations in HR, particularly when it is decreased; therefore, a formal adequacy assessment was performed to confirm the superior QT correction method for the study population [4, 15, 16].
The lack of a relevant effect of LCZ696 on cardiac repolarization is evidenced both by the absence of a relevant mean change in ∆∆QTcF, and the absence of outlier values during categorical change analyses. In addition, central tendency and categorical analyses of the prespecified ECG-derived PR interval and QRS duration did not show any clinically relevant treatment-related changes. The increased mean HR observed at 8 h following treatment with LCZ696 400 and 1200 mg compared with placebo or moxifloxacin was not dose related and was not temporally related to the maximum drug exposure. It may possibly be related to activity, as the earlier HR measurements on the assessment days were made during bed rest, while later measurements such as at 8 h post-dose were collected while the subjects were ambulant.
The PK analyses of LCZ696 revealed a dose proportional increase in the exposure of LBQ657 (the active metabolite of sacubitril), whereas the increase in the valsartan exposure was less than dose proportional, similar to the observations reported by Gu J et al. [3]. The observed mean Cmax for all three LCZ696 analytes (sacubitril, 7780 ng/mL; LBQ657, 40700 ng/mL; and valsartan, 9360 ng/mL) following the administration of a supratherapeutic dose of LCZ696 (1200 mg) in this study exceeded the highest exposures of LCZ696 analytes observed to date at steady state in subjects with severe renal impairment receiving LCZ696 400 mg once daily for 5 days (sacubitril, 4960 ng/mL; LBQ657, 30650 ng/mL; and valsartan, 5852 ng/mL) [17]. The mean maximum QTcF was noted at 1 h post-dose for both 400 and 1200 mg dose levels, similar to the median Tmax for sacubitril of 30 min for the 400 mg dose and 1 h for the 1200 mg dose. In contrast, the Tmax for LBQ657 and valsartan occurred at 2–3 h post-dose.
The upper bound of the two-sided 90 % CI of the model-predicted regression lines for the relationship between ∆∆QTcF and concentration of LCZ696 analytes at the supratherapeutic dose were below 10 ms, except for sacubitril, at concentrations >15000 ng/mL. However, the observed mean ∆∆QTcF values, corresponding to sacubitril concentrations >15000 ng/mL, remained below 10 ms. Sacubitril concentrations are not expected to exceed 15000 ng/mL upon the administration of single or multiple doses of LCZ696 400 mg (therapeutic dose). Even in subjects with severe renal impairment, the mean peak plasma concentration of sacubitril after multiple doses of LCZ696 400 mg once daily for 5 days was 4960 ng/mL [17], 3-fold lower than the highest individual Cmax of sacubitril following LCZ696 1200 mg administration in this study. Therefore, the model-predicted values for high exposures to sacubitril are not considered to be of clinical relevance.
LCZ696 (400 mg and 1200 mg) and moxifloxacin (400 mg) single doses were generally safe and well tolerated.
The overall safety profile in this study in healthy subjects was similar to the known safety profile of LCZ696 [18–20]. There were no repolarization changes on the ECGs and no AE reports that could suggest a potential for a pro-arrhythmic effect of LCZ696.
The major limitation of the present study is that the information was collected in healthy subjects and not in patients with HF, and that the supratherapeutic dose provides modest safety margins of 2.4-fold, 2.9-fold, and 2.0-fold, respectively, for the Cmax of sacubitril, LBQ657, and valsartan compared with patients with severe renal impairment.
Conclusions
Single therapeutic and supratherapeutic oral doses of LCZ696 (400 and 1200 mg) did not affect cardiac repolarization, as evidenced by the lack of effect on QTc interval. Moxifloxacin was associated with the expected prolongation of the QT interval, thereby demonstrating assay sensitivity. LCZ696 at 400 and 1200 mg doses was generally safe and well tolerated in this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(DOCX 23 kb)
(PDF 778 kb)
(PDF 403 kb)
Acknowledgments
The authors would like to thank Sreedevi Boggarapu, Novartis Healthcare Pvt. Ltd., Hyderabad, India, for providing writing/editorial assistance. All authors read and approved the final manuscript.
TL, PP, SV, IR JP, PJ, and PC contributed to the study design and/or data interpretation. PP participated in study design and statistical analysis. TL, JP, IR, and PC conducted the study.
The authors would like to thank Dr. Thomas Koernicke (Principle Investigator, PAREXEL International GmbH, Berlin, Germany) for study conduct and iCardiac Technologies Inc., (Rochester, NY, USA) for the input into the protocol development and analysis of ECG parameters.
Compliance with ethical standards
The study protocol was approved by the Ethics Committee of the study center. The study was designed and implemented in accordance with the ICH Guidelines for Good Clinical Practice E6 [11] and for Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs E14 [12], applicable local regulations, and the ethical principles laid down in the Declaration of Helsinki.
Conflict of interest
THL, PJ, PP, SV, and IR are employees of Novartis. JP and PC were employees of Novartis at the time this study was conducted.
References
- 1.1 Novartis Entresto™ (sacubitril and valsartan): US pescribing information. 2015. http://www.pharma.us.novartis.com. Accessed on 21 Nov 2015.
- 2.EMA. Entresto (sacubitril / valsartan) product information. (http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/004062/human_med_001929.jsp&mid=WC0b01ac058001d124). Last accessed on 12 Jan 2016.
- 3.Gu J, Noe A, Chandra P, Al-Fayoumi S, Ligueros-Saylan M, Sarangapani R, Maahs S, Ksander G, Rigel DF, Jeng AY, Lin TH, Zheng W, Dole WP. Pharmacokinetics and pharmacodynamics of LCZ696, a novel dual-acting angiotensin receptor-neprilysin inhibitor (ARNi) J Clin Pharmacol. 2010;50(4):401–414. doi: 10.1177/0091270009343932. [DOI] [PubMed] [Google Scholar]
- 4.Isbister GK, Page CB. Drug induced QT prolongation: the measurement and assessment of the QT interval in clinical practice. Br J Clin Pharmacol. 2013;76(1):48–57. doi: 10.1111/bcp.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wysowski DK, Corken A, Gallo-Torres H, Talarico L, Rodriguez EM. Postmarketing reports of QT prolongation and ventricular arrhythmia in association with cisapride and Food and Drug Administration regulatory actions. Am J Gastroenterol. 2001;96(6):1698–1703. doi: 10.1111/j.1572-0241.2001.03927.x. [DOI] [PubMed] [Google Scholar]
- 6.Cubeddu LX. Iatrogenic QT abnormalities and fatal arrhythmias: mechanisms and clinical significance. Curr Cardiol Rev. 2009;5(3):166–176. doi: 10.2174/157340309788970397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Redfern WS, Carlsson L, Davis AS, Lynch WG, MacKenzie I, Palethorpe S, Siegl PK, Strang I, Sullivan AT, Wallis R, Camm AJ, Hammond TG. Relationships between preclinical cardiac electrophysiology, clinical QT interval prolongation and torsade de pointes for a broad range of drugs: evidence for a provisional safety margin in drug development. Cardiovasc Res. 2003;58(1):32–45. doi: 10.1016/S0008-6363(02)00846-5. [DOI] [PubMed] [Google Scholar]
- 8.Klimas J, Kruzliak P, Rabkin SW. Modulation of the QT interval duration in hypertension with antihypertensive treatment. Hypertens Res. 2015;38(7):447–454. doi: 10.1038/hr.2015.30. [DOI] [PubMed] [Google Scholar]
- 9.Garg S, Narula J, Marelli C, Cesario D. Role of angiotensin receptor blockers in the prevention and treatment of arrhythmias. Am J Cardiol. 2006;97(6):921–925. doi: 10.1016/j.amjcard.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 10.Wang YH, Shi CX, Dong F, Sheng JW, Xu YF. Inhibition of the rapid component of the delayed rectifier potassium current in ventricular myocytes by angiotensin II via the AT1 receptor. Br J Pharmacol. 2008;154(2):429–439. doi: 10.1038/bjp.2008.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.ICH E6 (2015) Guidance for industry; good clinical practice: consolidated guidelines. 1996
- 12.Food, Drug Administration HHS International conference on harmonisation; guidance on E14 clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs; availability. Notice. Fed Reg. 2005;70(202):61134–61135. [PubMed] [Google Scholar]
- 13.Varro A, Baczko I. Cardiac ventricular repolarization reserve: a principle for understanding drug-related proarrhythmic risk. Br J Pharmacol. 2011;164(1):14–36. doi: 10.1111/j.1476-5381.2011.01367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hjalmarson A, Fagerberg B. MERIT-HF mortality and morbidity data. Basic Res Cardiol. 2000;95(Suppl 1):I98–I103. doi: 10.1007/s003950070017. [DOI] [PubMed] [Google Scholar]
- 15.Luo S, Michler K, Johnston P, Macfarlane PW. A comparison of commonly used QT correction formulae: the effect of heart rate on the QTc of normal ECGs. J Electrocardiol. 2004;37:81–90. doi: 10.1016/j.jelectrocard.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 16.Rowlands DJ. Graphical representation of QT rate correction formulae: an aid facilitating the use of a given formula and providing a visual comparison of the impact of different formulae. J Electrocardiol. 2012;45(3):288–293. doi: 10.1016/j.jelectrocard.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Ayalasomayajula S, Langenickel T, Chandra P, et al. Assessment of steady state pharmacokinetics of LCZ696 in patients with renal impairment. Clin Pharmacol Drug Dev. 2014;3:1–59. [Google Scholar]
- 18.Kario K, Sun N, Chiang FT, Supasyndh O, Baek SH, Inubushi-Molessa A, Zhang Y, Gotou H, Lefkowitz M, Zhang J. Efficacy and safety of LCZ696, a first-in-class angiotensin receptor neprilysin inhibitor, in Asian patients with hypertension: a randomized, double-blind, placebo-controlled study. Hypertension. 2014;63(4):698–705. doi: 10.1161/HYPERTENSIONAHA.113.02002. [DOI] [PubMed] [Google Scholar]
- 19.Ruilope LM, Dukat A, Bohm M, Lacourciere Y, Gong J, Lefkowitz MP. Blood-pressure reduction with LCZ696, a novel dual-acting inhibitor of the angiotensin II receptor and neprilysin: a randomised, double-blind, placebo-controlled, active comparator study. Lancet. 2010;375(9722):1255–1266. doi: 10.1016/S0140-6736(09)61966-8. [DOI] [PubMed] [Google Scholar]
- 20.McMurray JJV, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371(11):993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 23 kb)
(PDF 778 kb)
(PDF 403 kb)