Figure 3. The TLR-dependent ERC pathway and the TLR-independent ERGIC pathway cooperate in the formation of a cross-presenting phagosome.
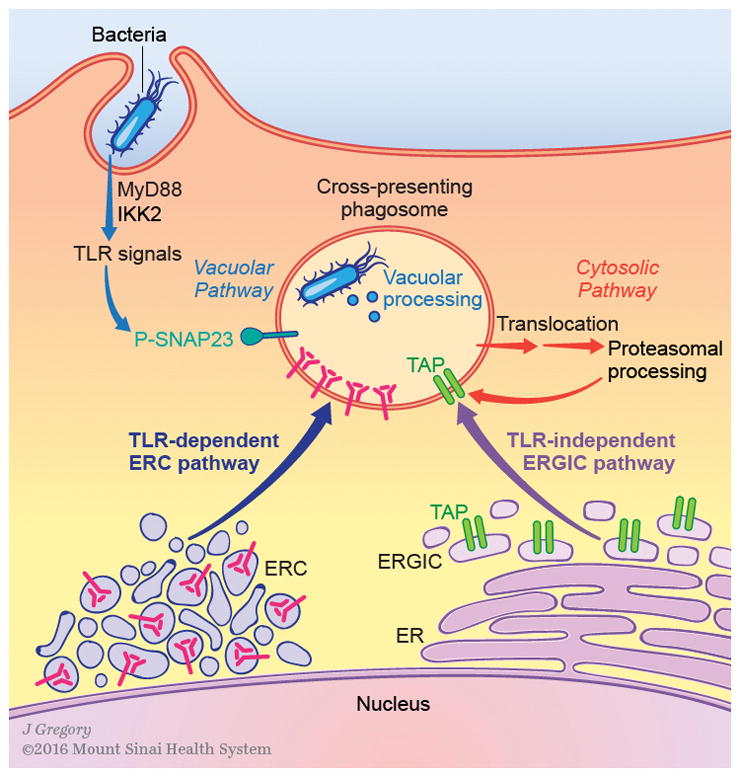
Upon phagocytosis of bacteria, two vesicular pathways are mobilized to greet the bacterium-containing phagosome. The pathway from the ERGIC is dependent on the ER SNARE Sec22b, and serves to deliver the peptide loading machinery including TAP, Tapasin, and Calreticulin. This pathway is mobilized independently of TLR signals, and the precise nature of the signals initiating its mobilization has not been defined. The pathway from the ERC is controlled by MyD88-IKK2 dependent TLR signaling which phosphorylates SNAP23 on phagosomes. Phospho-SNAP23 (pSNAP-23) flags microbial antigen containing phagosomes for receiving MHC-I molecules from the ERC in a TLR-dependent manner driven by a pSNAP-23 stabilized ERC-phagosome SNARE complex. Therefore, while delivery of the peptide loading complex occurs independently of TLR signals, delivery of MHC-I molecules is regulated by TLR signals. In the meantime, bacterial protein antigens derived from bacterial degradation are subjected to proteolysis through the vacuolar pathway which relies on the proteolytic activity of vacuolar proteases, or the cytosolic pathway which relies on cytosolic translocation of antigen, degradation by the proteasome, and transport back into phagosomes. The combined activity of these two pathways likely contributes to the diversity of peptides that can be generated from exogenous cargo and which in turn can be cross-presented by MHC-I molecules. Together with the vacuolar and cytosolic pathways of antigen processing, the ERC and ERGIC pathways of vesicular traffic equip phagosomes for cross-presentation of microbial antigen.
