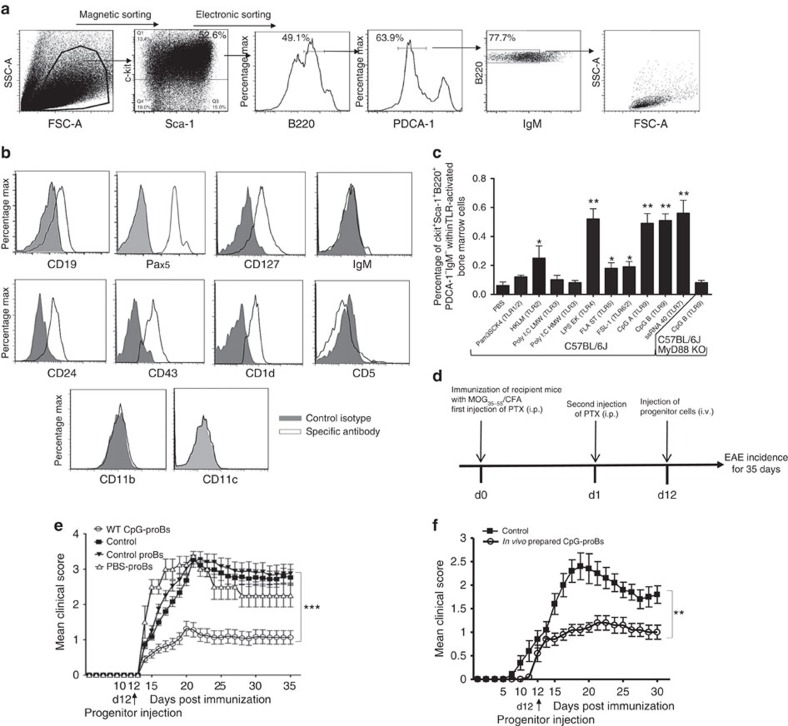
Figure 1. Phenotypic analysis of CpG-induced c-kit+Sca-1+B220+PDCA-1−IgM− BM cells and assessment of disease protection against ongoing EAE.
(a) BM cells incubated for 18 h with CpG-B (1 μg ml−1), were magnetically selected for c-kit+ cells, further labelled for Sca-1, B220, PDCA-1 and IgM and electronically sorted into small-size (FSClowSSClow) c-kit+Sca-1+B220+PDCA-1−IgM− cells. (b) Flow cytometry analysis of indicated B-cell markers expression by CpG-proB cells after cell-sorting as in a. (a,b) Cells were stained with specific antibodies (open histograms) or isotype controls (filled histograms). (c) Frequency of c-kit+Sca-1+B220+PDCA-1−IgM− cells emerging among BM cells after 18 h of incubation with different TLR agonists. CpG-B was tested in BM cell cultures of both WT and MyD88−/− C57BL/6J mice. Results are expressed as means±s.e.m. from three experiments. *P<0.05, **P<0.005 when comparing stimulated and unstimulated BM cells, using non-parametric Mann–Whitney's t-test. (d) Experimental protocol for MOG35–55 EAE disease induction and intravenous progenitor cell transfer (60,000 cells per mouse) at day 12 post-immunization. (e,f) EAE clinical scores (mean±s.e.m.) over 35 days of the indicated groups of mice. (e) n=30 mice per group, except for PBS-ProB-recipient group in which n=4 mice. (f) n=10 mice per group; ***P<0.001 when comparing control mice injected with PBS and recipients of WT CpG-proBs by two-way repeated measures ANOVA test. **P<0.005, between mice injected with in vivo prepared CpG-proBs and other groups, non significant between all other groups.