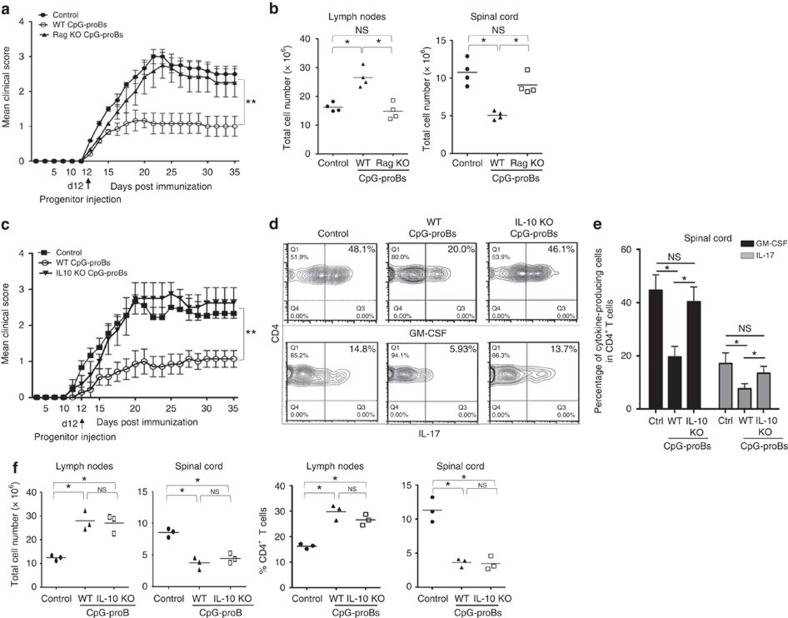
Figure 4. Role of maturation and IL-10 production capacities of CpG-proBs in the protection against EAE.
(a) EAE clinical scores (mean±s.e.m.) over 35 days of the indicated groups of mice. n=30 mice per group for control mice and for recipients of WT CpG-proBs, n=8 mice per group for recipients of Rag2−/−-derived CpG-proBs; ***P<0.001 when comparing control mice injected with PBS and recipients of WT CpG-proBs by two-way repeated measures ANOVA test. **P<0.005, between mice injected with WT CpG-proBs and other groups, non significant between all other groups. (b) Total cell number found in LNs and spinal cord of control mice and recipients of WT or Rag2−/− CpG-proBs (n=4 mice per group). (c) EAE clinical scores (mean±s.e.m.) in control mice or recipients of CpG-proB cells derived from the BM of WT or IL-10−/− mice. n=8 mice per group. **P<0.005, as assessed by two-way repeated measures ANOVA test, only between mice injected with WT CpG-proBs and other groups, non significant between all other groups. (d,e) Frequency of CD4+ T cells producing GM-CSF and IL-17 in the spinal cord of control mice with EAE, recipients of WT- or of IL-10−/−- CpG-proBs, shown in a representative experiment (d), and in three experiments (e) in which values are expressed as mean±s.e.m. (f) Total cell counts and frequency of CD4+ T cells recovered from reactive LN and spinal cord of control mice with EAE, or from recipients of WT- and IL-10−/− CpG-proBs (n=3 mice per group). *P<0.05 (Students't-test).