Figure 5.
UBQLN1 Conditionally Inhibits Mitochondrial Insertion of Ubiquitinated Clients
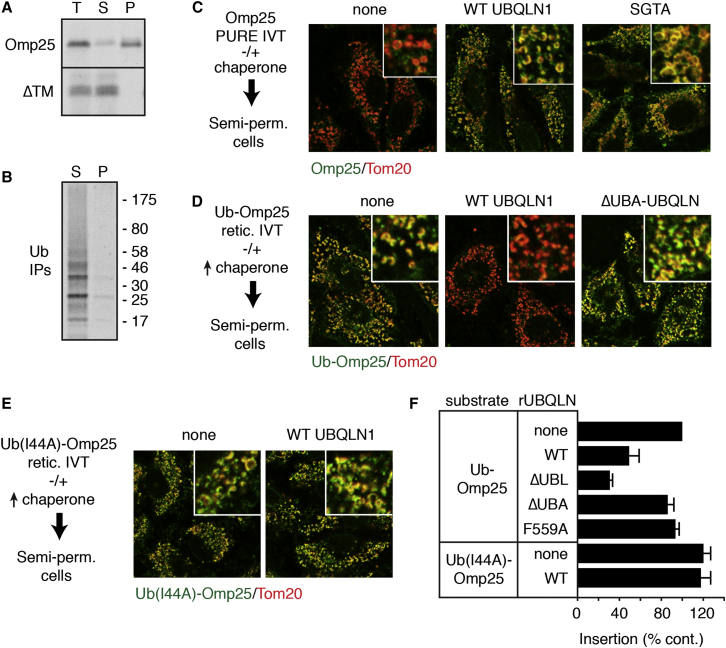
(A) Constructs containing the Omp25 TMD or a mutant disrupted in its hydrophobic domain (ΔTM) were translated in RRL in the presence of 35S-methionine and incubated with semi-permeabilized cultured cells. The total reactions (T) were fractionated into a soluble supernatant (S) and cell pellet (P), and the translated products were detected using autoradiography.
(B) A construct containing the Omp25 TMD was translated in a reaction containing 35S-methionine, His-tagged Ubiquitin, and semi-permeabilized cells. After translation, the reaction was fractionated as in (A), the ubiquitinated products in each fraction isolated via the His tag, and the ubiquitinated Omp25 detected using autoradiography.
(C) An HA-tagged construct containing the Omp25 TMD was translated in the PURE system supplemented without or with the indicated chaperone (either UBQLN1 or SGTA), and the products applied to semi-permeabilized cells. After washing, immunofluorescence microscopy was used to detect the localization of the translation product (green) relative to mitochondria (red).
(D) An HA-ubiquitin-tagged construct containing the Omp25 TMD was translated in RRL containing 1 μM of the indicated UBQLN1 protein. The translation products were applied to semi-permeabilized cells and visualized as in (C).
(E) As in (D), but with a construct containing the I44A mutation in Ubiquitin.
(F) The indicated substrates were translated in RRL supplemented with the indicated recombinant UBQLN1 proteins and analyzed for insertion into the mitochondria of semi-permeabilized cells as in (A). The graph shows the normalized quantification from three experiments (mean ± SD).
See also Figure S7.