Figure 2.
Crystal Structure of MINDY-1cat
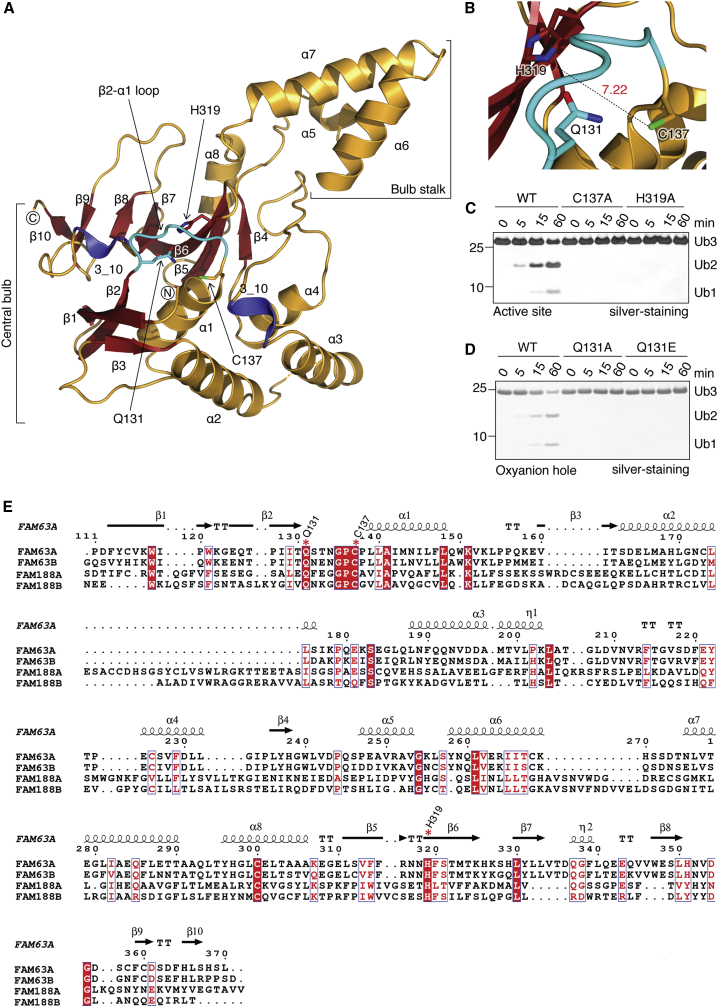
(A) Structure of catalytic domain of FAM63A/MINDY-1cat (110–370). The Cys loop (cyan) and the catalytic residues are indicated. β sheets are colored red and 3_10 helices blue.
(B) A close-up image of the MINDY-1cat catalytic site. Q131, C137, and H319 are shown.
(C and D) Hydrolysis of 1.9 μM K48-linked triUb by 1.6 μM MINDY-1 wild-type (WT) and the indicated mutants of the active site residues (C) or Q131 that forms the oxyanion hole residue (D).
(E) Sequence alignment of human FAM63A, FAM63B, FAM188A, and FAM188B. Secondary structure elements are shown for MINDY-1cat. The catalytic residues are highlighted with red asterisks. Residues 300–371 of FAM188A that form the EF hand domain have been omitted from the alignment. Fully conserved residues are shaded in red.
See also Figures S2 and S3.