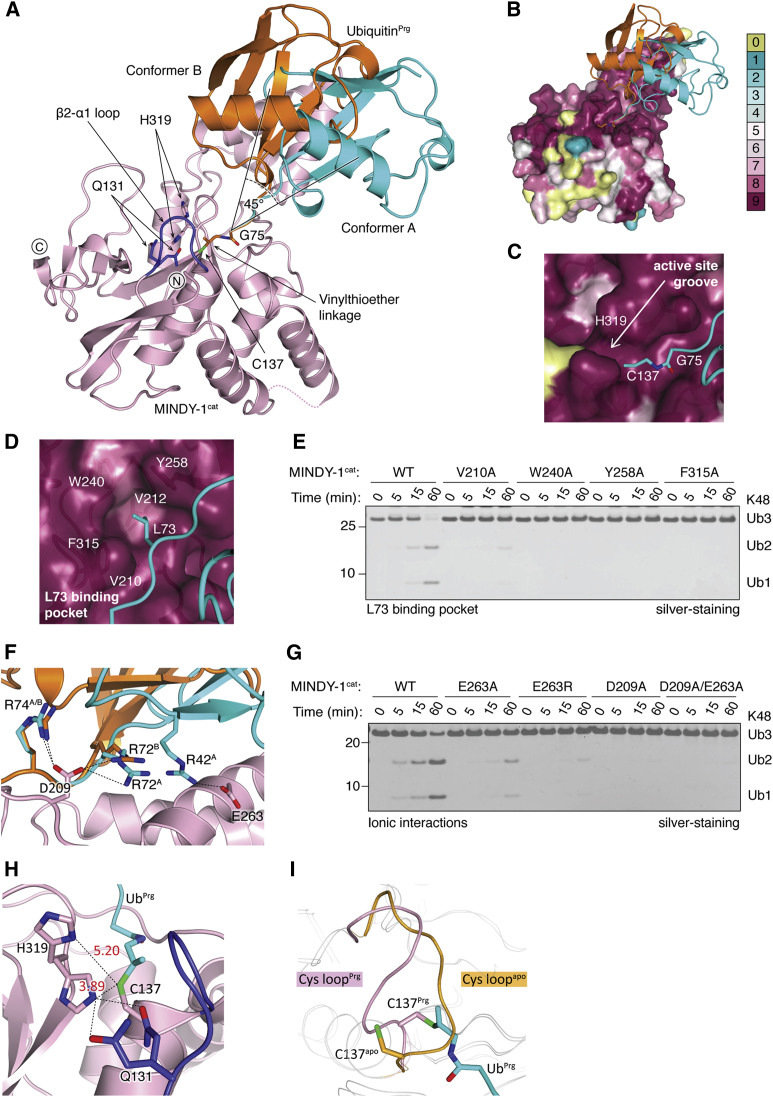
Figure 3.
Structure of MINDY-1cat∼Ub
(A) Overall structure of the catalytic core domain of MINDY-1 (pink) covalently bound to Ub. Ub exists in two alternate conformers in the structure that are rotated by ∼45° (cyan and orange). The vinylthioether linkage connecting UbPrg with MINDY-1 is shown in sticks. The Cys loop (β2-α1) is shown in blue.
(B) Conserved residues on the surface of MINDY-1 based on the sequence alignment in Figure S1C generated with the Consurf server (http://consurf.tau.ac.il) are shown. While the backside of MINDY-1cat is not conserved, surfaces interacting with and around the distal Ub are conserved.
(C) Close-up view of the catalytic groove where the C terminus of Ub sits, with coloring scheme as in (B).
(D) An aromatic cage formed by V212, W240, Y258, and F315 interacts with L73 of Ub. Close-up view of the conserved hydrophobic pocket accommodating L73 colored as in (B).
(E) DUB assays monitoring cleavage of 1.9 μM K48-triUb with 1.6 μM MINDY-1cat performed as in Figure 1C comparing activity of MINDY-1 and point mutants lining the L73 pocket: V210A, W240A, Y258A, and F315A.
(F) Close-up view of ionic interactions between Ub and MINDY-1.
(G) DUB assays comparing activity of MINDY-1 mutants that disrupt ionic interactions with Ub as performed in (E).
(H) Close-up image of the MINDY-1 catalytic triad showing two alternate conformations for H319 and Q131. Distances to C137 are indicated by dotted lines.
(I) Superposition of apo and complex states of MINDY-1cat shows movement of the Cys loop (apo in orange and MINDY-1cat∼Ub complex in pink).
See also Figure S4.