Figure 4.
MINDY-1 Cleaves PolyUb Chains in a Stepwise Manner
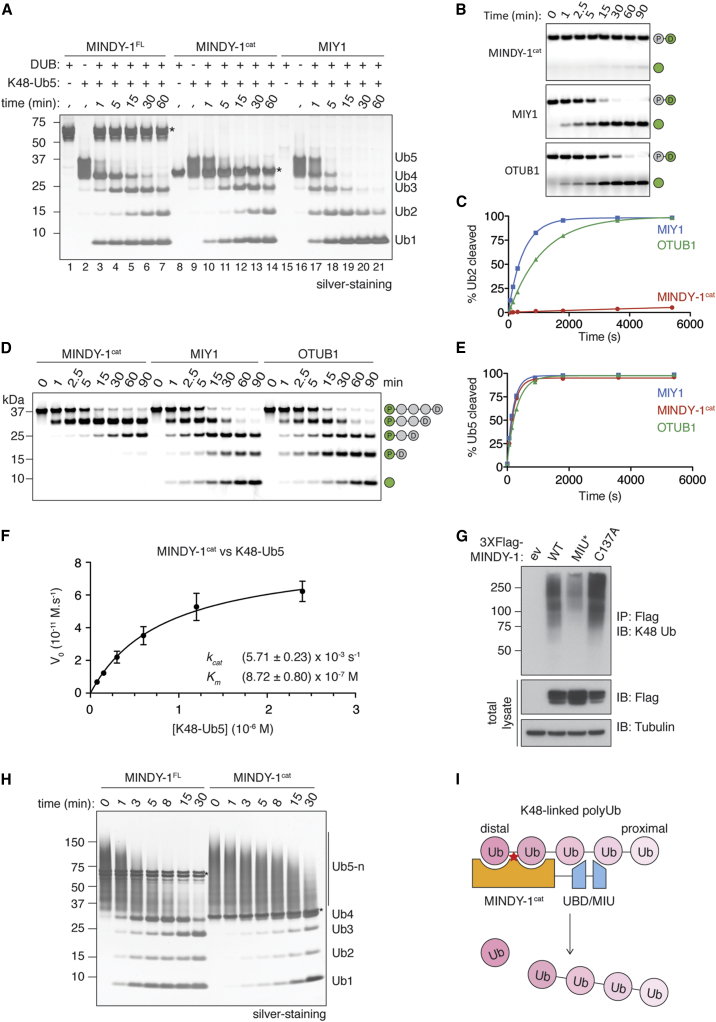
(A) Time course of cleavage of 3.5 μM K48-pentaUb by 1.6 μM of full-length MINDY-1 and MINDY-1cat and 160 nM MIY1. Asterisks indicate MINDY-1.
(B) Kinetics of cleavage of fluorescently labeled K48-linked diUb by MINDY-1cat, MIY1, and OTUB1. DUBs (1 μM) were incubated with 500 nM of K48-linked diUb that has been labeled with an infrared fluorescent dye at its distal Ub (green circle) for the indicated times. Fluorescent Ub was visualized using Odyssey LI-COR system at 800 nm channel. D, distal Ub, P, proximal Ub.
(C) Quantification of K48-Ub2 hydrolysis by MINDY-1cat, MIY1, and OTUB1 in (B). Percentage of the formed Ub1 intensity is shown on the y axis (n = 3; mean ± SD).
(D) DUB assays monitoring time-dependent cleavage of fluorescently labeled pentaUb by MINDY-1cat, MIY1, and OTUB1 as in (B). The proximal Ub of the chain (indicated by green circle) was labeled with an infrared fluorescent dye.
(E) Quantification of cleavage of K48-linked pentaUb by MINDY-1cat, MIY1, and OTUB1 in (D). The percentage of the total intensities of Ub4, Ub3, Ub2, and Ub1 formed is shown on the y axis (n = 3; mean ± SD). See also Figure S5.
(F) Steady-state kinetics of K48-linked pentaUb cleavage by MINDY-1cat. MINDY-1cat (15 nM) was incubated with 0.075–2.4 μM fluorescently labeled pentaUb (IR-K48-Ub5). The K48-Ub4 formed at the early time point (less than 10% of the substrate) was quantified to obtain initial velocities (V0). V0 was plotted against IR-K48-Ub5 concentration, and the data were fitted to the Michaelis-Menten equation to derive kcat and Km (n = 3; mean ± SD).
(G) Flag pull-downs from extracts of HEK293 cells inducibly expressing the indicated full-length MINDY-1 constructs. ev, empty vector; MIU∗, MIU mutant, L415A/A416G. Immunoblotting with a K48-linkage specific antibody was performed to monitor captured polyUb material.
(H) Time course comparing hydrolysis of K48-polyUb chains containing at least 5 Ub by full-length MINDY-1 and MINDY-1cat, which lacks the MIU.
(I) Model depicting the synergy between different domains of MINDY-1, where the UBD mediates substrate targeting to result in trimming of the Ub chain from the distal end by the catalytic domain.
See also Figure S5.