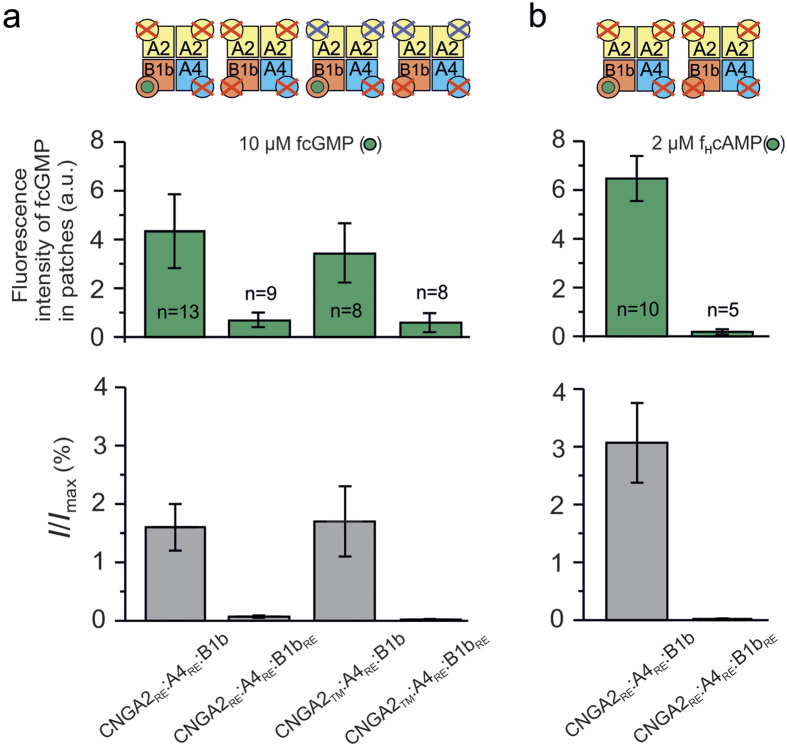
Figure 2. In a CNGA2:A4 background the CNGB1b subunit binds both fcGMP and fHcAMP and activates the channel.
(a) Binding of fcGMP to the CNGB1b subunit evokes channel activation. Binding of fcGMP to CNGB1b was observed when coexpressed with either CNGA2RE:A4RE or CNGA2TM:A4RE (upper diagram). When disabling the binding domain of CNGB1b, the fluorescence intensity is significantly reduced. Correspondingly, binding to CNGB1b in CNGA2RE:A4RE:B1b and CNGA2TM:A4RE:B1b channels evoked moderate channel activation which did not appear when the CNGB1b subunit was disabled (lower diagram). The bar graph shows the current amplitude at 10 μM fcGMP, I, corresponding to the binding experiments in the upper diagram, normalized to the current amplitude, Imax, recorded at 4 mM cGMP. (b) Binding of fHcAMP to CNGB1b was observed when coexpressed with both CNGA2RE and CNGA4RE. When disabling the binding domain of CNGB1b, the fluorescence intensity was nearly abolished. Correspondingly, CNGA2RE:A4RE:B1b channels were activated by the ligand only when the CNGB1b binding site was available.