Abstract
Primordial germ cell (PGC) specification early in development establishes the germline for reproduction and reproductive technologies. Germline replacement (GR) is a powerful tool for conservation of valuable or endangered animals. GR is achievable by germ cell transplantation into the PGC migration pathway or gonads. Blastula cell transplantation (BCT) can also lead to the chimeric germline containing PGCs of both donor and host origins. It has remained largely unknown whether BCT is able to achieve GR at a high efficiency. Here we report efficient GR by BCT into blastula embryos in the fish medaka (Oryzias latipes). Specifically, dnd depletion completely ablated host PGCs and fertility, and dnd overexpression remarkably boosted PGCs in donor blastulae. BCT between normal donor and host produced a germline transmission rate of ~4%. This rate was enhanced up to ~30% upon PGC boosting in donors. Most importantly, BCT between PGC-boosted donors and PGC-ablated hosts led to more than 90% fertility restoration and 100% GR. Therefore, BCT features an extremely high efficiency of fertility recovery and GR in medaka. This finding makes medaka an ideal model to analyze genetic and physiological donor-host compatibilities for BCT-mediated surrogate production and propagation of endangered lower vertebrates and biodiversity.
Sex and germline are the basis of reproduction, and their manipulation is essential for reproductive engineering and infertility treatment1,2,3. The germline is established by primordial germ cell (PGC) specification early in developing embryos of diverse animal species1,4,5,6. PGCs do not stay in the site of their formation but migrate through a somatic route into the developing gonad5. In adult animals, gonadal germ cells undergo meiosis and produce gametes, namely eggs in female and sperm in male6,7. Germline replacement (GR) is a powerful reproductive technique and holds enormous potential in maintenance and propagation of scientifically or commercially valuable animals and more importantly, in restoration and propagation of endangered species even from cryopreserved germ cells or germline-competent cells for biodiversity conservation. GR is achieved by transplantation of donor cells into hosts, leading to the production of germline chimeras capable of producing donor-derived gametes for germline transmission to next generations. Surrogate reproduction by transplantation between different transplantation donor and host is an emerging paradigm for restoration and propagation of endangered species. In fish, Yoshizaki et al. first reported surrogate production of rainbow trout in salmon hosts2,8.
Germ cell transplantation (GCT) has so far been the gold standard for GR in diverse organisms such as fish. Multiple GCT procedures have been developed with respect to germ cell origin, source and treatment, donor-host combination, developmental stage, place and treatment of host. Yoshizaki and his colleagues have pioneered GCT in salmonids by using normal or triploid embryonic gonads as hosts and reported several important scientific advances and innovative procedures for GR in surrogate production2,8,9,10. Procedures have also been established for GCT into busulfan-sterilized host gonads of maturing or matured tilapia11 and atherinopsids12, for GCT into the embryonic peritoneal cavity as part of the PGC migration pathway in jack mackerel13 and tilapia14, and into blastula host in medaka15. An attractive alternate to GCT is blastula/blastocyst cell transplantation (BCT). The inner cell mass cells of mammalian blastocyst embryos are pluripotent and competent for PGC formation even after transplantation into host blastocysts. Embryonic stem (ES) cells are similar to the ICM and have widely been used for the production of germline chimeras via blastocyst transplantation. In zebrafish, BCT produces germline chimeras at a low efficiency by using normal blastula embryos16,17 but at a high efficiency in dnd depleted blastula embryos18. BCT is also able to produce germline chimeras in loach19.
The laboratory fish medaka (Oryzias latipes) is an excellent lower vertebrate model to analyze and manipulate embryonic development20. We make use of this fish for reproductive biology and biotechnologies. Medaka has diploid embryonic stem (ES) cells capable of somatic chimera production, haploid ES cells capable of whole animal production21, male germ stem cells capable of test-tube production7 and transgenic lines for visualization of PGCs and adult germ cells22,23. Medaka PGCs from early embryonic cells can be specified in culture22 and are able to colonize the host germline upon blastula transplantation15. PGC preformation appears to operate in medaka24 as in zebrafish25. However, germ plasm components in medaka do not localize as in zebrafish25,26 but rather distribute widely27,28. The fate and number of PGC precursors are determined by dnd whose RNA forms particles and asymmetrically segregates into 8 blastomeres of 32–64 cleavage embryos. Altering the level of dnd expression can greatly alter the PGC number, ranging from remarkable increase by nearly 3-folds to complete absence of PGC specification1. In addition, medaka is the best studied lower vertebrate for sex determination29,30,31, initiation32 and maintenance33.
We are developing the BCT as a necessary complementary approach to GCT for GR in basic and applied research. Previously, we and others have established procedures and parameters for the efficient production of somatic and germline chimeras via BCT34,35,36 and ES cell transplantation21,34,35,37. Here we report the BCT procedure and parameters for 100% GR. We show that dnd depletion is 100% efficient for host sterilization via abolishing PGC formation, and that dnd overexpression remarkably enhances the efficiency of germline chimera formation via increasing the PGC number in donor blastula cells. Strikingly, BCT between PGC-boosted donor and PGC-ablated host leads to a 90% high efficiency of fertility restoration and 100% GR. This finding makes medaka an ideal model organism to analyze biological and technical donor-host compatibilities for BCT-mediated surrogate production and propagation of endangered lower vertebrates and their biodiversities.
Results
Donor strain and treatment
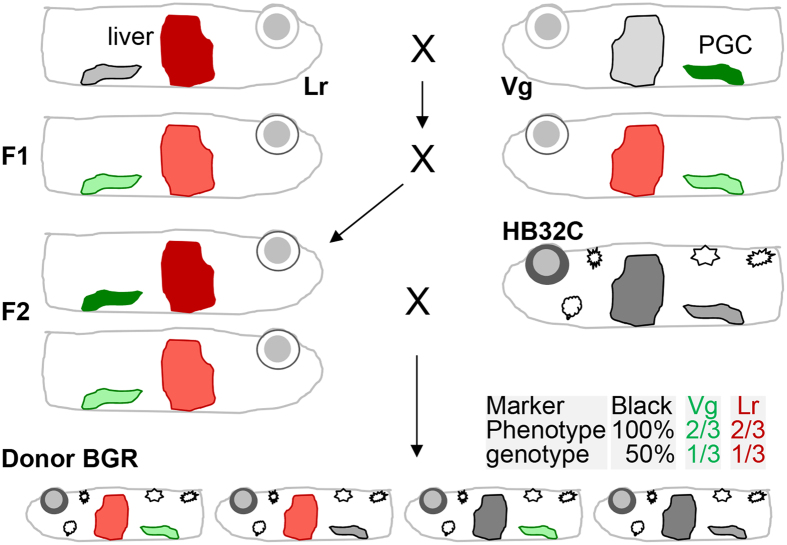
The ability to assess the GR efficiency by BCT is the availability of easily visible markers capable of distinguishing donor and host contribution to germline transmission. We generated BGR donor embryos that offer 3 dominant traits, namely wild-type melanocytes for black pigmentation, GFP-labeled germ cells (Vg) and RFP-labeled liver (Lr for liver red). BGR is 100% phenotypically pigmented and genetically heterozygous (50%) for wild-type melanophores, and 66.6% positive for GFP-labeled PGCs or RFP-labeled liver but 33.3% for Vg or Lr transgenes according to the breeding scheme (Fig. 1). Embryo observations confirmed the predicted percent values of the phenotypes (Table 1).
Figure 1. Preparation of donor BGR.
Lr and Vg are transgenic i3 albino fishes that express RFP in the liver (Lr for liver red) and GFP in germ cells (Vg). F2 fish positive for both Vg and Lr from the cross between Lr and Vg were mated with wild-type (black) pigmentation strain HB32C. The resultant BGR embryos and fish were all heterozygous for black pigmentation (stars) but only 2/3 positive for either Lr (red) or Vg (green).
Table 1. Phenotypes of donor and chimeric embryos1.
| Sample | Embryos observed | Pigmented, n (%) | Lr-positive, n (%)2 | Vg-positive PGC |
Total PGCs predicted4 |
|||
|---|---|---|---|---|---|---|---|---|
| n (%)2 | PGC3 | % | PGC3 | |||||
| BGR donor | normal | 31 | 31 (100) | 20 (64.5) | 21 (67.7) | 33.1 ± 2.93 | 100 | 33.1 ± 2.93 |
| dnd RNA | 30 | 30 (100) | 20 (66,6) | 20 (66.7) | 82.5 ± 5.42 | 100 | 82.5 ± 5.42 | |
| Chimera5 | Class I | 36 | 36 (100) | 21 (58.3) | 17 (47.2) | 1.41 ± 0.51 | 63.5 | 1.6 ± 0.8 |
| Class II | 32 | 32 (100) | 18 (56.2) | 26 (81.2) | 2.08 ± 0.74 | 92.4 | 2.7 ± 1.3 | |
| Class III | 37 | 37 (100) | 21 (56.8) | 30 (81.1) | 2.07 ± 0.78 | |||
| Class IV | 24 | 24 (100) | 18 (75.0) | 19 (79.2) | 2.21 ± 0.71 | |||
1Vg was observed at stages 18–23 when PGCs were positioned bilaterally to somites and easily countable. Melanocyte and Lr were observed from 5 dpf onwards. All embryos are positive for pigmentation.
2Comparisons between embryos observed and positive for Lr or Vg.
3Number of heterozygous VgPGCs per embryos presented as means ± sd.
4Percentages of donor PGC-containing embryos and numbers of total PGCs. These values in donor embryos are the same as experimentally determined, and in chimeras were predicted from the genotype of BGR embryos (see Fig. 1) and the bimodal distribution by using 1 PGC including 0.66 VgPGC [2/3(33.1 PGCs) per 1000-cell donor blastula; class I) and 2.5 PGCs including 1.65 VgPGCs [2/3(82.5 PGCs) per 1000-cell donor blastula; classes II-IV] as the input numbers of PGCs within 30 donor blastula cells transplanted.
5For chimera classes see Fig. 2.
In GCT, the majority if not all of donor germ cells are capable of contributing to the host germline. In BCT, only a minority of donor cells have this capability. In medaka, for example, a blastula embryo has approximately 1000 cells including 33 PGCs1,22,38. The standard BCT procedure transplants approximately 30 blastula cells and thus just one single PGC on average into each blastula host, and the proportional contribution by donor PGCs will be only 3% of the germline of resultant chimeras. In order to enhance the PGC proportion in donor blastula cells, BGR embryos were injected at the 1-cell stage with 100 pg of dnd:ch mRNA and examined for the PGC number at the blastula stage. A non-injected control BGR embryo on average was found to have 33.1 PGCs, and this number was increased by 2.5-folds to 82.5 (Table 1). Notably, the generation of more PGCs by dnd RNA injection at the 1-cell stage did not alter the percentage values of phenotypic traits. Thus, the PGC proportion in donor embryos can greatly be increased by dnd overexpression.
Host strain and treatment
We used medaka strain i3 as the BCT host. This strain displays albinism and does not contain any transgene. The host was treated in two ways to favor germline chimera formation. Previously we have shown that γ-irradiation can compromise hosts to increase donor cell contribution into the liver and gonad34, and that dnd depletion can completely prevent PGC specification1. Both approaches were adopted for host treatment. Host embryos were exposed to γ-irradiation at 4–8 cell stages or injected at the 1-cell stage with 20~100 pg of MOdnd, an antisense morpholino oligo capable of specifically inhibiting medaka dnd mRNA translation1. Control and treated embryos were grown into adulthood and examined for fertility by progeny test. As shown in Table 2, approximately 98.3% (n = 59) of adults from control embryos were fertile; γ-irradiation dramatically reduced the survival rate from 44.7% of control embryos to 9.8% and produced 94.6% fertile adults; and dnd depletion by injecting MOdnd did not affect the survival rate but severely reduced % fertility at 20 pg (32.2%, n = 31) or completely abolished fertility (100%) at 50 pg (n = 49) and 100 pg (n = 98). Thus, γ-irradiation is inefficient for host sterilization, whereas dnd depletion is highly efficient for host sterilization via completely abolishing PGCs.
Table 2. Survival and fertility of hosts and chimeras1.
| Type | Treatment | Embryo sampled | Adult, n (%)2 | Fertile, n (%)3 |
|---|---|---|---|---|
| i3 host | Control | 132 | 59 (44.7) | 58 (98.3) |
| γ-irradiation (6 gy) | 379 | 37 (9.8) | 35 (94.6) | |
| MOdnd (20 pg) | 73 | 31 (42.5) | 10 (32.2) | |
| MOdnd (50 pg) | 121 | 49 (40.5) | 0 (0) | |
| MOdnd (100 pg) | 226 | 98 (43.4) | 0 (0) | |
| Chimera4 | Class I | 156 | 56 (35.9) | 56 (100) |
| Class II | 173 | 61 (35.2) | 60 (98.4) | |
| Class III | 188 | 58 (32.2) | 53 (91.4) | |
| Class IV | 154 | 13 (8.4) | 12 (92.3) |
1Fish from embryos with or without BCT were maintained for testing fertility, the ability to produce F1 progeny embryos, from 3 months post hatching onwards by massive breeding. Fertility was judged by egg production for females and egg-fertilizing ability for males. Massive breeding was performed first on experimental fish for one month, followed by 2 weeks of breeding with added fertility-proven HdrR males and another 2 weeks of breeding with added fertility-proven HdrR females. Fertile fish were randomly chosen for progeny test (see Table 3).
2This is the survival rate by comparison between embryos samples and adults obtained.
3This is the fertility rate by comparison between total adults obtained and fertile adults.
4Chimeras produced by transplanting ~30 blastula cells into each blastula host. Classes I-IV are BCT between normal donor and host (class I), dnd RNA-injected (100 pg) donor and normal (class II), MOdnd-injected (100 pg; class III) and γ-γ-irradiated hosts (class IV). For more details see Fig. 2.
Blastula cell transplantation
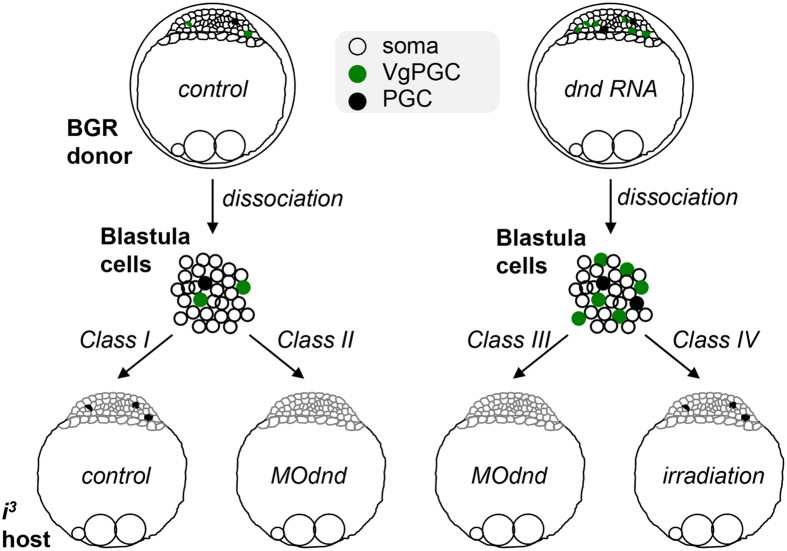
We performed 4 classes of BCT on the basis of donor-host combinations (Fig. 2). Class I is BCT between normal donor and host, class II between dnd-overexpressing donor and normal host, class III between dnd-overexpressing donor and dnd-depleted host, and class IV between dnd-overexpressing donor and γ-irradiated host. In each class, approximately 30 blastula cells were transplanted into the deep cells of a blastula host. It was found that chimeras of classes I-IV were 100% positive for pigmented melanophores of donor origin (Table 2), demonstrating the successful and reproducible BCT into hosts of an albino background.
Figure 2. Donor and host treatment and BCT scheme.
BGR donor embryos at the 1-cell stage were not injected (control) or injected with 100 pg of dnd:ch RNA (dnd RNA) and dissociated at the midblastula stage into single blastula cells. Albino i3 host embryos at the 1-cell stage were not injected (control) or injected with 20–100 pg of dnd-targeting morpholino oligo MOdnd (MOdnd) and dechorionated for BCT at the midblastula stage. Alternatively, Host embryos at the 4–8-cell stages were subjected to gamma-irradiation at 6 gy and used for BCT at the midblastula stage. Approximately 30 blastula cells were transplanted into the deep cells of a blastula host. BCT were performed between normal donor and host (class I), dnd RNA-injected donor and normal (class II), MOdnd-injected (100 pg; class III) and irradiated hosts (class IV). Note that dnd RNA increases PGCs in donor blastula cells and that MOdnd abolishes PGCs in the host blastulae.
We made use of PGCs to precisely evaluate the chimera frequency and chimerism degree (Table 1; Table S1). The former refers to % chimera production, and the latter to the number or proportion of donor-derived cells as a precise measure for relative donor contribution in chimeras. The initial number of PGCs produced by an embryo is well-known in medaka and can easily be counted during somitogenesis when PGCs are positioned bilaterally to somites along the axis. A total of 36 class-I chimeras were examined, 21 and 17 of them were found to be positive for Lr and Vg, respectively, producing chimera frequencies of 58.3% and 47.2%. The 17 Vg-positive chimeras had 1.35 Vg-PGCs on average, thus a chimerism degree of 1.35. When calibrated to 66.7% for phenotypically VgPGCs, the chimera frequency and chimerism degree were predicted to be 63.5% and 1.56 for total PGCs. Since a control blastula embryo produces 33.1 PGCs on average, the 1.56 donor PGCs will have 5% proportional contribution to the PGC pool of class-I chimeras. Therefore, class-I BCT is able to produce a low efficiency of germline chimera production.
Dnd-overexpression increased the PGC number per embryos by 2.5 folds from 33.1 in control embryos to 82.5 in the dnd RNA-injected embryo. Transplantation of such PGC-boosted donor blastula cells into control hosts (class-II BCT) significantly increased the chimeras frequency and chimerism degree to 81.2% and 2.04 for Vg, the values become 92.4% and 2.66 upon extrapolation to total PGCs (compare class I and II; Table 2). On the other hand, host treatment by γ-irradiation or dnd depletion did not alter the chimera frequency and chimerism degree (compare class II and III or IV). Notably, pretreatments of donor and host embryos had no significant effect on somatic chimera production. One exception to this was an increased Lr chimera frequency to 75% in γ-irradiated hosts compared with 56.2–58.3% in control and dnd RNA-injected hosts (Table 1), which is consistent with our previous observation that γ-irradiation promotes donor ES cell’s contribution to the liver34. Taken together, the use of dnd-overexpressing donor blastula cells remarkably increases the input number of donor PGCs and thus the germline chimera frequency and chimerism degree without affecting somatic chimera formation, and the use of dnd-depleted hosts does not alter the input number of donor PGCs and thus the formation of germline chimeras without altering somatic chimera formation.
Donor PGC migration and proliferation
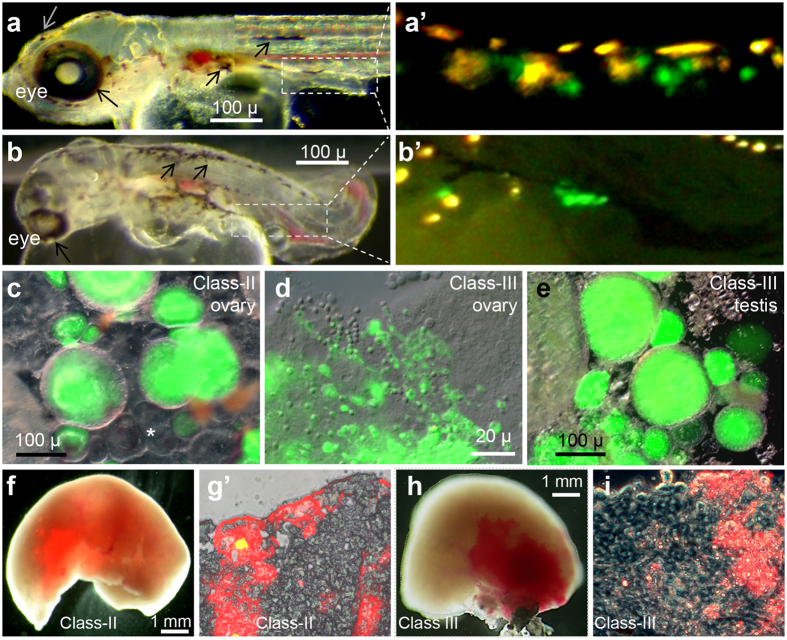
BCT-introduced donor PGCs were able to migrate into the embryonic gonad and increased their number. On average, a chimera of classes II-IV could receive ~2.5 PGCs or ~2 VgPGCs (because only 2/3 of PGCs is Vg-positive). The majority of classes II-IV chimeras indeed contained 1~3 VgPGCs in the migratory route at earlier stages. Notably, many such chimeras had 2~5 VgPGCs in the gonad, as shown for representatives from class-III chimeras (Fig. 3a,a’) and class-IV chimeras having 7 VgPGCs each (Fig. 3b,b), suggesting proliferation of donor PGCs during and post migration.
Figure 3. Chimeric fry and adult organs.
(a and a’) Class-III chimera, showing normal development and donor-derived melanocytes (arrows) on the body surface and PGCs (green) in the gonad. (b and b’) Class-IV chimera, showing developmental defects (bent body, curved tail and abnormal eye) and donor-derived melanocytes (arrows) on the body surface and PGCs (green) in the gonad. (c) Flat-mounted ovary of a class-II chimera, showing few GFP-positive oocytes and many GFP-negative oocytes (asterisk). (d,e) Flat-mounted testis (d) and ovary (e) of class-III chimeras, showing GFP-positive germ cells (green). (f,g) Liver of a class-II chimera, showing RFP-positive cells in the liver and its flat-mounted part. (h,i) Liver of a class-III chimera, showing RFP-positive cells in the liver and its flat-mounted part.
Germline transmission
In order to evaluate germline transmission, donor fish and BCT-derived chimeras were grown into adulthood and tested for their fertility by breeding as described above for the host. Chimeras of classes I and II from BCT into normal hosts exhibited fertility rates of 100% and 98.4% fully comparable to 98.3 for non-transplanted hosts (Table 2). Notably, chimeras of classes III from BCT into dnd-depleted hosts exhibited a reduced fertility rate of 91.4%, which suggests that transplantation of 30 dnd-overexpressing blastula cells containing 2.5 PGCs is able to restore the fertility in more than 90% of chimeras (Table 2). The fertility rate value 91.4% is similar to 92.4% as the germline chimera frequency predicted from the BCT conditions by the binorminal distribution (Table 2). In addition, chimeras of classes IV from BCT into γ-irradiated hosts also exhibited a reduced fertility rate of 92.3%.
Fertility-proven donor fish and chimeras were subjected to progeny test via crossing with i3, and resultant embryos and fry were observed for pigmentation, liver and gonad. Among 8 donor fish, 8, 6 and 5 were found to be transmitters of pigmentation, Vg and Lr. They produced 494 F1 progeny embryos, of which 48.6%, 33.2% and 33.6% were positive for black pigmentation, Vg and Lr (Table 3, Table S2), which are in accordance with 50% for the pigmentation locus and 33.3% for the Vg and Lr transgenes in BGR donor embryos. Doubling of 48.6% and tripling of 33.2% and 33.6% led to genotype-calibrated transmission rates 97.2%, 99.6% and 100.8% for pigmentation, Vg and Lr, producing an average of 99.2% as the genotype-calibrated general germline transmission rate for the donor (Table 3).
Table 3. Germline transmission of donor and chimeras1.
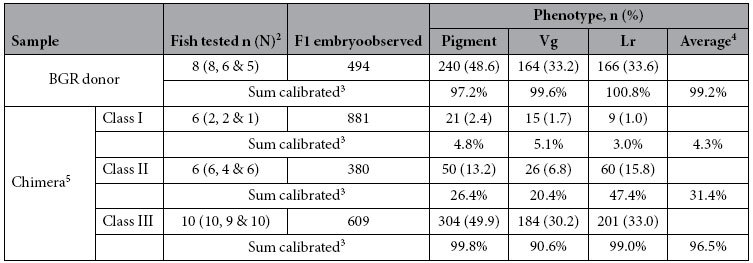
1Fertile male and female after fertility test (see Table 1) were randomly chosen for test cross with albino host i3 of opposite sexes, and F1 embryos were examined for Vg, Lr and pigmentation phenotypes.
2n, total number of fish individuals tested; N, numbers of germline transmiters for pigment, Vg and Lr.
3Percentage germline transmission was calibrated to 50% (heterozygosity) for pigment and to 1/3 for Vg and Lr by doubling the observed percentage values for pigment and tripling the observed percentage values for Vg and Lr, respectively.
4Average of calibrated percentage values for pigment, Vg and Lr.
Among 6 class-I chimeras progeny-tested, 2, 2 and 1 were found to be transmitters of pigmentation, Vg and Lr. They produced 881 F1 progeny embryos, of which 2.4%, 1.7% and 1% were positive for black pigmentation, Vg and Lr, generating an average of 4.3% as the genotype-calibrated general germline transmission rate for class-I chimeras (Table 3, Table S2). Among 6 class-II chimeras progeny-tested, 6, 4 and 6 were found to be transmitters of pigmentation, Vg and Lr. They produced 380 F1 progeny embryos, of which 13.2%, 6.8% and 15.8% were positive for black pigmentation, Vg and Lr, giving rise to an average of 31.4% as the genotype-calibrated general germline transmission rate for class-II chimeras (Table 3, Table S2). Comparing 31.4% of class-II chimeras with 4.3% of class-I chimeras revealed a 7.3-fold enhancement in germline transmission rate. Taken together, PGC boosting in donor embryos by dnd overexpression is able to greatly increase germline chimera production.
A total of 10 class-III chimeras were progeny-tested. Among them, 10, 9 and 10 were found to be transmitters of pigmentation, Vg and Lr. They produced 609 F1 progeny embryos, of which 49.9%, 30.2% and 33%% were positive for black pigmentation, Vg and Lr. Calibration to the donor genotype led to an average of 96.5% as the general germline transmission rate for class-III chimeras (Table 3, Table S2). This value is comparable to 99.2% of the donor. It deserves to note that one of the 10 chimeras incapable of transmitting Vg was a male capable of transmitting pigmentation at 52.5% and Lr at 40,7% to its F1 progeny (serial number 6, Table S2), demonstrating that its germline is of donor origin. Taken together, germ cells in fertile class-III chimeras are exclusively of donor origin, and the combination between PGC boosting by dnd expression in the donor and PGC ablation by dnd depletion in the host allows for a high efficiency of germline chimera formation and 100% GR in medaka.
Chimeric liver and gonads of germline chimeras
We examined the chimerism degree in adult organs of progeny-tested chimeric Vg and Lr transmiters of classes III and III. In the ovary of a typical class-II chimera, GFP-positive female germ cells of donor origin coexisted with many GFP-negative counterparts largely of host origin (Fig. 3c). In the testis and ovary of class-III chimeras, the proportion of GFP-positive germ cells was remarkably enhanced (Fig. 3d,e). In the liver of both class-II and -III chimeras, RFP-negative and –positive cells were easily detectable in the whole organ and its squash (Fig. 3f–i). These results together with fertility data, donor genotypes and phenotypes as well as chimeric germline transmission demonstrate that BCT between dnd-overexpression donor and dnd-depleted host is able to achieve complete GR without affecting somatic chimerism.
Discussion
In this study, we have developed a BCT procedure that allows for the generation of fertile fish invariantly with the germline of completely donor origin. On one hand, we show that PGC-depleted embryos invariantly develop into germ cell-less sterile adults, whereas 91.4% of class-III chimeras from PGC-depleted hosts transplanted with 30 PGC-boosted blastula cells containing 2.5 PGCs develop into fertile females and males. Since the value of 91.4% is indifferent from 92.4% predicted as the frequency of PGC transplantation from the binorminal distribution, the introduction of 1 or few donor PGCs into the majority – if not all - of chimeras is able to restore fertility. On the other hand, there is no difference in germline transmission and phenotypic segregation between chimeras and donor fish, demonstrating the absence of host germline. In summary, the BCT procedure reported in this study is able to restore fertility in PGC-deficient embryos at an extremely high efficiency and ensure that all fertile chimeras generate gametes of donor origin, a crucial factor for restoration and propagation of biodiversity.
Our success is attributed to the combinational use of two approaches. One is 100% PGC elimination in the host by dnd depletion. Prevention of PGC formation and interference with PGC migration or survival in subsequent development may compromise and abolish the germline and fertility at varying efficiencies. We have recently reported dnd as the PGC specifier in medaka and its depletion prevents PGC formation1. PGC elimination by compromising PGC migration or survival has been achieved by dnd depletion in several fish species including zebrafish3,18,39,40, loach41, goldfish42, starlet43 and Atlantic salmon44. This study corroborates and extends these reports by demonstrating the ability of dnd depletion for 100% host sterilization during embryonic and adult development.
The other approach to the 100% GR is ascribed to the new addition of boosting PGCs in the donor by dnd overexpression. One of major challenges for BCT-mediated GR is that only a small proportion of blastula cells are PGCs or PGC-competent cells capable of germline contribution. In this study we show that dnd overexpression is able to elevate the PGC proportion from ~3.3% to ~8.2%, in accordance with our previous report that dnd is not only necessary for PGC specification but also sufficient for increasing PGCs via boosting PGC precursors in medaka1. More importantly, the use of PGC-boosted donor blastula cells is indeed able to increase the efficiency of germline chimera production by 7 folds. Identification of factors capable of remarkably increasing the PGC number will be valuable for efficient GR by BCT. It has been reported that overexpression of several germ genes such as vasa and piwi is generally unable to increase the PGC number in zebrafish45 and medaka22,38. There are two exceptions. One is the zebrafish buc whose overexpression is capable of increasing the PGC number by up to ~50% 46. The other is dnd in medaka as shown here and previously1. The ability of dnd to boost PGCs has so far been limited to medaka, as its overexpression in zebrafish does not increase the PGC number26.
GCT and BCT feature their respective advantages and are perfectly complementary each other for different applications. GCT is of choice for the propagation of scientifically or commercially valuable but rare or super animals. In this case, embryos are either rare and thus limited in number or longer available (e.g. the embryo that has giving rise to a super animal) for donor blastula cell preparation, whereas biopsies may be taken to enable germ cell preparation and propagation into a large for surrogate production of numerous female and male progeny via GCT. Consequently, surrogate progeny from a single super animal may establish a self-mating population to further increase the individual number by normal sexual reproduction. Since the progeny could originate from a single genotype, the self-mating system will minimize genetic variations arising from meiotic segregation and assortment and thus maximize the probability to reconstitute the genotype and phenotype of a super animal.
BCT is, on the other hand, of choice particularly for biodiversity conservation via restoration and propagation of endangered species. There are three key factors essential for surrogate production of biodiversity, namely donor-host immunocompatibility, purity and diversity of progeny animals. BCT perfectly fulfil these requirements. There is no immune rejection in developing fish embryos as has been well-demonstrated by the production of intra-species chimeras17,18,21,35,37,47 and even interordinal chimeras48. Easy and reliable PGC ablation by dnd depletion ensures GR-dependent fertility restoration and thus a pure donor origin of progeny from BCT chimeras. As for diversity, donor blastula cells may be collected from hundreds and thousands of PGC-boosted blastulae and thus rich in genetic diversity. BCT may introduce PGCs from different donor embryos into the chimeric germline. Consequently, PGCs in different BCT chimeras and even one and the same chimera may differ in individuality and thus genotype, which makes an apparent sense in terms of effective population size (Ne), a parameter essential for biodiversity conservation and management. In practice, a Ne of 100 is considered as the minimal value necessary for biodiversity restoration and maintenance. If a population has 50 males and 50 females, then Ne is 100 [(4 × 50 × 50)/(50 + 50)] according to the equation Ne = (4NmNf)/(Nm + Nf), where Ne is the effective population size; Nm is the number of males; and Nf is the number of females. In this study we have shown that medaka BCT chimeras often have ~2.5 PGCs of different origins and thus genotypes. Consequently, ~20 male and ~20 female BCT chimeras will approach this minimal Ne value. Blastula cells are easy for dissociation and testing for viability by observation of pseudopodial formation and cell division and do not need to characterize the cell identity as they either contain or produce PGCs in diverse animals1,4,5,6. Blastula hosts are also easy for preparation, cell transplantation and judgement of successful BCT via monitoring donor cells’ behaviors during subsequent development34,35,49. BCT is particularly ideal for surrogate production of animals that have high fecundity and robust embryology, a feature common to many lower vertebrates such as frogs and fish. In addition, BCT is the only strategy to preserve and propagate scientifically valuable mutants capable of blastula formation but die from abnormal subsequent development or incapable of normal gametogenesis in a somatically defect gonad, as has been illustrated in zebrafish18.
Several fundamental questions remain to be answered before surrogate production can be used in conservation practice. These include donor-host phylogeny and developmental-physiological compatibility in gametogenesis and fertilization. Our finding that BCT can achieve a high efficiency of fertility restoration and GR makes medaka an ideal model organism for the experimental analysis of these biological parameters towards BCT-mediated surrogate production and propagation of endangered lower vertebrates and biodiversity.
Methods
Chemicals and fish
Chemicals were purchased from Sigma, enzymes were from New England Biolabs, and PCR reagents were from TaKaRa unless otherwise indicated. Fish work was performed in strict compliance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Advisory Committee for Laboratory Animal Research in Singapore and approved by this committee (Permit Number: 27/09). Medaka strains HB32C, i3, HdrR and transgenic lines (see below) were maintained under an artificial photoperiod of 14-h light to 10-h darkness at 26 °C as described34.
Morpholino oligo and plasmids
MOdnd and pCSdnd:chDD were described1, the former is morpholino antisense oligo targeting the medaka dnd mRNA around the translational initiation, the latter expresses a fusion between the medaka Dnd protein and cherry protein. pLFABP-rfp contains the 2.8-kb liver-specific promoter of the zebrafish liver fatty acid binding protein and drives RFP expression specifically in embryonic and adult liver in zebrafish50 and medaka34.
Microinjection
Medaka embryos were injected at the 1-cell stage with 20~50 pg of MOdnd or 100 pg of capped dnd:ch mRNA synthesized from pCSdnd:chDD as described1,22,51.
Cell transplantation
Preparation of donor blastula cells, dechorionated host MBEs and cell transplantation were performed essentially as described21,22,34. MBEs of albino strain i3 were used as the transplantation host. The transplantation donor was generated in multiple steps as follows. HB32C is a wild-type pigmentation strain52. Lr is a liver-red transgenic line of strain orange produced by microinjecting pLFABP-rfp. Vg is a transgenic line of see-through medaka Wakamatsu et al., 2001 that expresses GFP from the medaka Vasa promoter specifically in germ cells22. Crossing between Lr and Vg led to F1 fish that expresses GFP in germ cells and RFP in the liver on the i3 albino background. F1 fish positive for Vg and Lr were mated to produce F2 generation. F2 embryos positive for Vg and Lr were grown to adults designated as GL fish that were homozygous (1/3) or heterozygous (2/3) for Vg and Lr transgenes and thus 66.6% for Vg and Lr phenotypes. Finally, GL females were crossed with HB32C male, generating BGL embryos that were used as the transplantation donor. BGR is heterozygous (50%) to black melanophores responsible for wild-type pigmentation and 33.3% for Vg and Lr transgenes and phenotypes. In some experiments, donor embryos at the 1-cell stage were injected with 100 pg of dnd:ch RNA to increase the PGC number, and host embryos also at the 1-cell stage were injected with 20–100 ng of MOdnd to ablate PGCs or at the 4- to 8-cell stages were subjected to γ-irradiation at 6 gy34.
Fertility test
Adult fish from MOdnd-injected i3 embryos were first maintained on their own for 1 month to observe egg production. They were then maintained for 2 weeks in the presence of fertile HdrR males and another 2 weeks in the presence of fertile HdrR females. Adult chimeras were examined similarly by using fertile HdrR males and fertile HdrR females as the testers. Fertile females were proven by egg-laying. Fertile males were proven by the ability to fertilize eggs in pair-wise mating.
Germline transmission
Fertile male and female chimeras were individually test-crossed with i3 albino medaka. Progeny were phenotypically analyzed for the donor contribution by pigmentation, GFP and RFP expression.
Histology of chimeras
The liver, ovary and testis of progeny-tested chimeras were dissected and examined for donor cell derivatives by GFP and RFP expression.
Microscopy
Observation and photography on Leica MZFIII stereo microscope, Zeiss Axiovertinvert and Axiovert upright microscopes with a Zeiss AxioCam M5Rc digital camera (Zeiss Corp) were as described previously21,28,34.
Statistics
Statistical analyses were calculated by using Graphad Prism v4.0. Data consolidated were presented as mean ± s.d. and p values were calculated by using non-parametric student’s t-test. Genetic data were evaluated by using chi-square test.
Additional Information
How to cite this article: Li, M. et al. Germline replacement by blastula cell transplantation in the fish medaka. Sci. Rep. 6, 29658; doi: 10.1038/srep29658 (2016).
Supplementary Material
Acknowledgments
We thank Jiaorong Deng for fish breeding, Veronica Wong and Choy Mei Foong for laboratory management. This work was supported by grants to M.L. from the National Natural Science Foundation of China (31372520) and Shanghai Pujiang Program (15PJ1403100) and to Y.H. from the National Research Foundation of Singapore (NRF-CRP7-2010-03).
Footnotes
Author Contributions M.L. and N.H. designed the study. M.L., N.H. and H.X. did research. J.S. and Y.H. wrote the paper.
References
- Hong N. et al. Dnd Is a critical specifier of primordial germ cells in the medaka fish. Stem Cell Reports 6, 411–421 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi Y., Yoshizaki G. & Takeuchi T. Biotechnology: surrogate broodstock produces salmonids. Nature 430, 629–630 (2004). [DOI] [PubMed] [Google Scholar]
- Tzung K. W. et al. Early depletion of primordial germ cells in zebrafish promotes testis formation. Stem Cell Reports 4, 61–73 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz E. Primordial germ-cell development: the zebrafish perspective. Nat Rev Genet 4, 690–700 (2003). [DOI] [PubMed] [Google Scholar]
- Wylie C. Germ cells. Cell 96, 165–174 (1999). [DOI] [PubMed] [Google Scholar]
- Xu H., Li M., Gui J. & Hong Y. Fish germ cells. Sci China Life Sci 53, 435–446 (2010). [DOI] [PubMed] [Google Scholar]
- Hong Y. et al. Establishment of a normal medakafish spermatogonial cell line capable of sperm production in vitro. Proc Natl Acad Sci USA 101, 8011–8016 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okutsu T., Shikina S., Kanno M., Takeuchi Y. & Yoshizaki G. Production of trout offspring from triploid salmon parents. Science 317, 1517 (2007). [DOI] [PubMed] [Google Scholar]
- Okutsu T., Suzuki K., Takeuchi Y., Takeuchi T. & Yoshizaki G. Testicular germ cells can colonize sexually undifferentiated embryonic gonad and produce functional eggs in fish. Proc Natl Acad Sci USA 103, 2725–2729 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Iwasaki Y., Shikina S. & Yoshizaki G. Generation of functional eggs and sperm from cryopreserved whole testes. Proc Natl Acad Sci USA 110, 1640–1645 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacerda S. M. et al. A new and fast technique to generate offspring after germ cells transplantation in adult fish: the Nile tilapia (Oreochromis niloticus) model. PLoS One 5, e10740 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majhi S. K., Hattori R. S., Rahman S. M. & Strussmann C. A. Surrogate production of eggs and sperm by intrapapillary transplantation of germ cells in cytoablated adult fish. PLoS One 9, e95294 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita T. et al. Functional Sperm of the Yellowtail (Seriola quinqueradiata) Were Produced in the Small-Bodied Surrogate, Jack Mackerel (Trachurus japonicus). Mar Biotechnol (NY) 17, 644–654 (2015). [DOI] [PubMed] [Google Scholar]
- Farlora R. et al. Intraperitoneal germ cell transplantation in the Nile tilapia Oreochromis niloticus. Mar Biotechnol (NY) 16, 309–320 (2014). [DOI] [PubMed] [Google Scholar]
- Li Z., Li M., Hong N., Yi M. & Hong Y. Formation and cultivation of medaka primordial germ cells. Cell Tissue Res 357, 71–81 (2014). [DOI] [PubMed] [Google Scholar]
- Kawakami Y. et al. Generation of germ-line chimera zebrafish using primordial germ cells isolated from cultured blastomeres and cryopreserved embryoids. Int J Dev Biol 54, 1493–1501 (2010). [DOI] [PubMed] [Google Scholar]
- Lin S., Long W., Chen J. & Hopkins N. Production of germ-line chimeras in zebrafish by cell transplants from genetically pigmented to albino embryos. Proc Natl Acad Sci USA 89, 4519–4523 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciruna B. et al. Production of maternal-zygotic mutant zebrafish by germ-line replacement. Proc Natl Acad Sci USA 99, 14919–14924 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa M., Kobayashi T. & Ueno K. Production of germline chimera in loach (Misgurnus anguillicaudatus) and proposal of new method for preservation of endangered fish species. J Exp Zool 293, 624–631 (2002). [DOI] [PubMed] [Google Scholar]
- Wittbrodt J., Shima A. & Schartl M. Medaka–a model organism from the far East. Nat Rev Genet 3, 53–64 (2002). [DOI] [PubMed] [Google Scholar]
- Yi M., Hong N. & Hong Y. Generation of medaka fish haploid embryonic stem cells. Science 326, 430–433 (2009). [DOI] [PubMed] [Google Scholar]
- Li M. et al. Medaka vasa is required for migration but not survival of primordial germ cells. Mech Dev 126, 366–381 (2009). [DOI] [PubMed] [Google Scholar]
- Tanaka M., Kinoshita M., Kobayashi D. & Nagahama Y. Establishment of medaka (Oryzias latipes) transgenic lines with the expression of green fluorescent protein fluorescence exclusively in germ cells: a useful model to monitor germ cells in a live vertebrate. Proc Natl Acad Sci USA 98, 2544–2549 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herpin A. et al. Specification of primordial germ cells in medaka (Oryzias latipes). BMC Dev Biol 7, 3 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon C., Kawakami K. & Hopkins N. Zebrafish vasa homologue RNA is localized to the cleavage planes of 2- and 4-cell-stage embryos and is expressed in the primordial germ cells. Development 124, 3157–3165 (1997). [DOI] [PubMed] [Google Scholar]
- Weidinger G. et al. dead end, a novel vertebrate germ plasm component, is required for zebrafish primordial germ cell migration and survival. Curr Biol 13, 1429–1434 (2003). [DOI] [PubMed] [Google Scholar]
- Shinomiya A., Tanaka M., Kobayashi T., Nagahama Y. & Hamaguchi S. The vasa-like gene, olvas, identifies the migration path of primordial germ cells during embryonic body formation stage in the medaka, Oryzias latipes. Dev Growth Differ 42, 317–326 (2000). [DOI] [PubMed] [Google Scholar]
- Xu H., Li Z., Li M., Wang L. & Hong Y. Boule is present in fish and bisexually expressed in adult and embryonic germ cells of medaka. PLoS One 4, e6097 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda M. et al. DMY is a Y-specific DM-domain gene required for male development in the medaka fish. Nature 417, 559–563 (2002). [DOI] [PubMed] [Google Scholar]
- Nanda I. et al. A duplicated copy of DMRT1 in the sex-determining region of the Y chromosome of the medaka, Oryzias latipes. Proc Natl Acad Sci USA 99, 11778–11783 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura T. et al. Sex determination. foxl3 is a germ cell-intrinsic factor involved in sperm-egg fate decision in medaka. Science 349, 328–331 (2015). [DOI] [PubMed] [Google Scholar]
- Zhang X. et al. Autosomal gsdf acts as a male sex initiator in the fish medaka. Sci Rep 6, 19738 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuyama H. et al. Dmrt1 mutation causes a male-to-female sex reversal after the sex determination by Dmy in the medaka. Chromosome Research 20, 163–176 (2012). [DOI] [PubMed] [Google Scholar]
- Hong N. et al. Accessibility of host cell lineages to medaka stem cells depends on genetic background and irradiation of recipient embryos. Cell Mol Life Sci 67, 1189–1202 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Y., Winkler C. & Schartl M. Efficiency of cell culture derivation from blastula embryos and of chimera formation in the medaka (Oryzias latipes) depends on donor genotype and passage number. Dev Genes Evol 208, 595–602 (1998). [DOI] [PubMed] [Google Scholar]
- Wakamatsu Y. et al. Generation of germ-line chimeras in medaka (Oryzias latipes). Molecular Marine Biology and Biotechnology 2, 325–332 (1993). [Google Scholar]
- Hong Y., Winkler C. & Schartl M. Production of medakafish chimeras from a stable embryonic stem cell line. Proc Natl Acad Sci USA 95, 3679–3684 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Hong N., Gui J. & Hong Y. Medaka piwi is essential for primordial germ cell migration. Curr Mol Med 12, 1040–1049 (2012). [DOI] [PubMed] [Google Scholar]
- Wong T. T. & Zohar Y. Production of reproductively sterile fish by a non-transgenic gene silencing technology. Sci Rep 5, 15822 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. et al. A controllable on-off strategy for the reproductive containment of fish. Sci Rep 5, 7614 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto T. et al. Sexual dimorphism of gonadal structure and gene expression in germ cell-deficient loach, a teleost fish. Proc Natl Acad Sci USA 107, 17211–17216 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto R. et al. Germ cells are not the primary factor for sexual fate determination in goldfish. Dev Biol 370, 98–109 (2012). [DOI] [PubMed] [Google Scholar]
- Linhartova Z. et al. Sterilization of sterlet Acipenser ruthenus by using knockdown agent, antisense morpholino oligonucleotide, against dead end gene. Theriogenology 84, 1246–1255 e1 (2015). [DOI] [PubMed] [Google Scholar]
- Wargelius A. et al. Dnd knockout ablates germ cells and demonstrates germ cell independent sex differentiation in Atlantic salmon. Sci Rep 6, 21284 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaut H., Pelegri F., Bohmann K., Schwarz H. & Nusslein-Volhard C. Zebrafish vasa RNA but not its protein is a component of the germ plasm and segregates asymmetrically before germline specification. J Cell Biol 149, 875–888 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bontems F. et al. Bucky ball organizes germ plasm assembly in zebrafish. Curr Biol 19, 414–422 (2009). [DOI] [PubMed] [Google Scholar]
- Hong N., He B. P., Schartl M. & Hong Y. Medaka embryonic stem cells are capable of generating entire organs and embryo-like miniatures. Stem Cells Dev 22, 750–757 (2013). [DOI] [PubMed] [Google Scholar]
- Hong N. et al. Interordinal chimera formation between medaka and zebrafish for analyzing stem cell differentiation. Stem Cells Dev 21, 2333–2341 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Y. & Schartl M. Establishment and growth responses of early medakafish (Oryzias latipes) embryonic cells in feeder layer-free cultures. Mol Mar Biol Biotechnol 5, 93–104 (1996). [Google Scholar]
- Korzh S. et al. Requirement of vasculogenesis and blood circulation in late stages of liver growth in zebrafish. BMC Dev Biol 8, 84 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Y., Winkler C., Liu T., Chai G. & Schartl M. Activation of the mouse Oct4 promoter in medaka embryonic stem cells and its use for ablation of spontaneous differentiation. Mech Dev 121, 933–943 (2004). [DOI] [PubMed] [Google Scholar]
- Hong Y., Winkler C. & Schartl M. Pluripotency and differentiation of embryonic stem cell lines from the medakafish (Oryzias latipes). Mech Dev 60, 33–44 (1996). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.