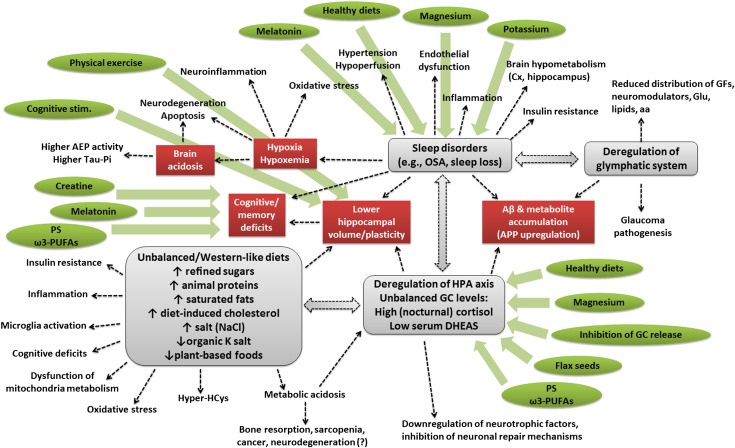
FIGURE 1.
Schematic representation of the complex network underlying the onset of cognitive impairment and eventually AD. Imbalanced, Western-like, high-fat diets elicit deregulation of the HPA axis and of GC (e.g., cortisol) release and lower hippocampal volumes, besides inducing a plethora of other effects (e.g., insulin resistance, inflammation, microglia activation, cognitive impairments, dysfunction of mitochondria metabolism, oxidative stress, hyperhomocysteinemia, and metabolic acidosis), which typically characterize type 2 diabetes, metabolic syndrome, and AD. Deregulation of cortisol release can affect sleep quality, reduce hippocampal volume, promote the accumulation of Aβ plaques and other metabolites, downregulate the synthesis of neurotrophic factors, and inhibit neuronal repair mechanisms. Sleep disorders may themselves compromise cortisol release; reduce hippocampal volume and plasticity; induce cognitive and memory deficits; elicit hypoxia and hypoxemia (which is responsible for increased brain acidosis, neuronal degeneration, inflammation, oxidative stress, induction of asparaginyl endopeptidase activity, and increase of phospho-tau); increase hypertension and hypoperfusion, endothelial dysfunctions, inflammation, cortical and hippocampal hypometabolism, and insulin resistance; and reduce the functionality of the glymphatic system. Deregulations of the glymphatic system can cause accumulation of Aβ plaques and other metabolites; reduce the distribution of growth factors, neuromodulators, glucose, lipids, and amino acids; and can contribute to glaucoma pathogenesis. Some dietary interventions and supplements (indicated in green), such as plant-based and Mediterranean (healthy) diets, PS and ω-3 PUFAs, melatonin, creatine, magnesium, potassium, flax seed cultivars, and inhibitors of GC release, in combination with cognitive stimulation and physical exercise, can soothe ≥1 of these risk factors and, for this reason, might be considered as nonpharmacologic interventions aimed at preventing the risk of AD or reducing its symptoms. Aβ, amyloid β AD, Alzheimer disease; AEP, asparaginyl endopeptidase; APP, amyloid precursor protein; Cx, cortex; DHEAS, dehydroepiandrosterone sulfate; GC, glucocorticoid; GF, growth factor; Glu, glucose; HPA, hypothalamic-pituitary-adrenal; Hyper-HCys, hyperhomocysteinemia; OSA, obstructive sleep anea; PS, phosphatidylserine.