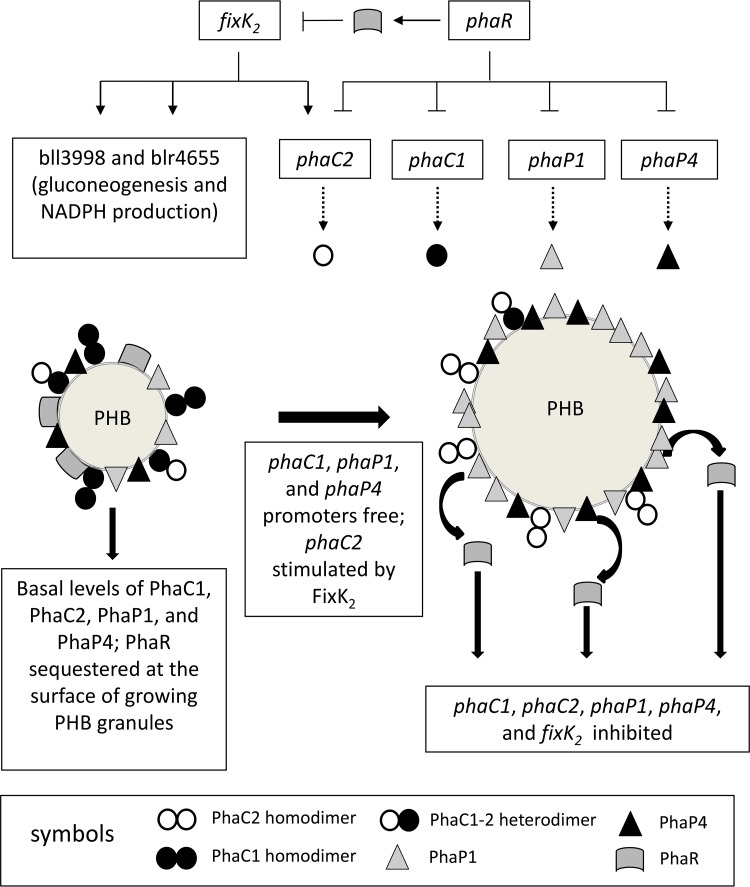
FIG 5.
Proposed model for the regulation of PHB synthesis in B. diazoefficiens, including the possible role of FixK2 (→, stimulation; ⫞, inhibition; solid lines indicate transcription; dashed lines depict translation). Permissive conditions, e.g., low O2, lead to an increase of NADPH and acetyl-CoA concentrations; thus, PHB biosynthesis starts using these metabolites as the substrates. This biosynthetic process is catalyzed initially by high levels of 3-ketoacyl-CoA thiolase, acetoacetyl-CoA reductase, and PHB synthase due to PhaR sequestration at the PHB granule surface. Meanwhile, phaC2 expression is stimulated by FixK2, increasing the relative PhaC2/PhaC1 concentration, which progressively decreases total PhaC activity during PHB granule growth. As granules grow, less PHB surface becomes available for PhaR and PhaP, which may provoke a competitive displacement of PhaR by PhaP from the surface that in turn may repress PhaR-controlled promoters. In the phaR mutant, these promoters are not repressed; therefore, PhaP1, PhaP4, and FixK2 levels are increased. Hence, FixK2 stimulates the expression of phaC2 and the biosynthesis of EPS. Altogether, these changes result in low levels of PHB in the phaR mutant. Moreover, in the ΔphaP1 ΔphaP4 strain, the very large PHB granule devoid of PhaP1 and PhaP4 may be able to allocate more PhaR than the wild type at the beginning of PHB biosynthesis. This PhaR sequestration may promote more PHB biosynthetic activity, leading to higher PHB accumulation before the PhaC2/PhaC1 ratio reaches its critical value and PhaR begins to accumulate in the cytoplasm. Protein symbols are indicated at the bottom of the figure. For more details, see the text.