Abstract
BACKGROUND
The prevalence of dementia is expected to soar as the average life expectancy increases, but recent estimates suggest that the age-specific incidence of dementia is declining in high-income countries. Temporal trends are best derived through continuous monitoring of a population over a long period with the use of consistent diagnostic criteria. We describe temporal trends in the incidence of dementia over three decades among participants in the Framingham Heart Study.
METHODS
Participants in the Framingham Heart Study have been under surveillance for incident dementia since 1975. In this analysis, which included 5205 persons 60 years of age or older, we used Cox proportional-hazards models adjusted for age and sex to determine the 5-year incidence of dementia during each of four epochs. We also explored the interactions between epoch and age, sex, apolipoprotein E ε4 status, and educational level, and we examined the effects of these interactions, as well as the effects of vascular risk factors and cardiovascular disease, on temporal trends.
RESULTS
The 5-year age- and sex-adjusted cumulative hazard rates for dementia were 3.6 per 100 persons during the first epoch (late 1970s and early 1980s), 2.8 per 100 persons during the second epoch (late 1980s and early 1990s), 2.2 per 100 persons during the third epoch (late 1990s and early 2000s), and 2.0 per 100 persons during the fourth epoch (late 2000s and early 2010s). Relative to the incidence during the first epoch, the incidence declined by 22%, 38%, and 44% during the second, third, and fourth epochs, respectively. This risk reduction was observed only among persons who had at least a high school diploma (hazard ratio, 0.77; 95% confidence interval, 0.67 to 0.88). The prevalence of most vascular risk factors (except obesity and diabetes) and the risk of dementia associated with stroke, atrial fibrillation, or heart failure have decreased over time, but none of these trends completely explain the decrease in the incidence of dementia.
CONCLUSIONS
Among participants in the Framingham Heart Study, the incidence of dementia has declined over the course of three decades. The factors contributing to this decline have not been completely identified. (Funded by the National Institutes of Health.)
Dementia is the leading cause of dependence and disability in the elderly population worldwide.1–3 As the average life expectancy increases, the prevalence of dementia4 and associated monetary costs are expected to increase exponentially.5 A few studies have suggested that the age-specific incidence of dementia (i.e., the risk of dementia at any specific age) might be decreasing, but these studies either have shown a trend that failed to reach significance6,7 or have relied on comparisons of prevalence data that were ascertained at multiple time points.8–10 One study showed no decline in incidence.11 Temporal trends are best derived through continuous monitoring for new cases in a representative community-based sample over an extended observation period, with the use of consistent diagnostic criteria; however, such data from published studies are limited. We estimated temporal trends in the incidence of dementia over three decades among participants in the Framingham Heart Study.
METHODS
STUDY DESIGN
The Framingham Heart Study is a community-based, longitudinal cohort study that was initiated in 1948. The original cohort comprised 5209 residents of Framingham, Massachusetts, and these participants have undergone up to 32 examinations, performed every 2 years, that have involved detailed history taking by a physician, a physical examination, and laboratory testing.12 In 1971, a total of 5214 offspring of the participants in the original cohort and the spouses of these offspring were enrolled in an offspring cohort. The participants in the offspring cohort have completed up to 9 examinations, which have taken place every 4 years.13
All participants have provided written informed consent. Study protocols and consent forms were approved by the institutional review board at the Boston University Medical Center.
SURVEILLANCE FOR DEMENTIA
Surveillance methods have been published previously,14,15 and further details about dementia tracking are provided in the Supplementary Appendix (available with the full text of this article at NEJM.org). Cognitive status has been monitored in the original cohort since 1975, when comprehensive neuropsychological testing was performed. At that time, participants with low cognitive scores (the lowest 10%) also underwent neurologic assessment, and then a dementia-free inception cohort was established that included all dementia-free persons in the entire cohort.16 Since 1981, participants in this cohort have been assessed at each examination with the use of the Mini–Mental State Examination (MMSE)17; participants are flagged for further cognitive screening if they have scores below the prespecified cutoffs, which are adjusted for educational level and prior performance. Participants in the offspring cohort have undergone similar monitoring18; they answered a subjective memory question in 1979, have undergone serial MMSEs since 1991, and have taken a 45-minute neuropsychological test every 5 or 6 years since 1999. Participants who are identified as having possible cognitive impairment19 on the basis of these screening assessments are invited to undergo additional, annual neurologic and neuropsychological examinations. If two consecutive annual evaluations show reversion toward normal cognition, participants are returned to the regular tracking pool. Additional examinations are also performed when subjective cognitive decline is reported by the participant or a family member, either spontaneously between examinations or during annual health-status updates; on referral by a treating physician or by ancillary investigators of the Framingham Heart Study; or after review of outside medical records.14
A dementia review panel, which includes a neurologist and a neuropsychologist, has reviewed every case of possible cognitive decline and dementia ever documented in the Framingham Heart Study. For cases that were detected before 2001, a repeat review was completed after 2001 so that up-to-date diagnostic criteria could be applied. The panel determines whether a person had dementia, as well as the dementia subtype and the date of onset, using data from previously performed serial neurologic and neuropsychological assessments, telephone interviews with caregivers, medical records, neuroimaging studies, and, when applicable and available, autopsies.15 After a participant dies, the panel reviews medical and nursing records up to the date of death to assess whether the participant might have had cognitive decline since his or her last examination.20
The diagnosis of dementia is based on criteria from the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV).21 The diagnosis of Alzheimer’s disease is based on criteria for possible, probable, or definite Alzheimer’s disease from the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINCDS–ADRDA).22,23 The diagnosis of vascular dementia is based on criteria for possible or probable vascular dementia from the National Institute of Neurological Disorders and Stroke and the Association Internationale pour la Recherche et l’Enseignement en Neurosciences (NINDS–AIREN).24 The diagnostic algorithm allows participants to have more than one subtype of dementia.
EDUCATIONAL LEVEL AND VASCULAR RISK FACTORS
In the Framingham Heart Study, extensive information is collected at each examination. For this analysis, educational level was dichotomized (high school diploma vs. no high school diploma). Vascular risk factors were assessed, including systolic and diastolic blood pressures, use of antihypertensive medications, body-mass index, current smoking status, diabetes status, lipid levels, use of lipid-lowering agents, apolipoprotein E (APOE) ε4 status, and a history of cardiovascular events, including stroke and transient ischemic attacks, coronary heart disease, heart failure, and peripheral arterial disease; further details are provided in the Methods section of the Supplementary Appendix.
STATISTICAL ANALYSIS
We evaluated four nonoverlapping epochs, which started at the beginning of the second, fourth, sixth, and eighth examination cycles for the offspring cohort and the four closest corresponding time periods for the original cohort. The baseline examination period was between 1977 and 1983 for the first epoch, between 1986 and 1991 for the second epoch, between 1992 and 1998 for the third epoch, and between 2004 and 2008 for the fourth epoch. We selected these dates to maximize the person-years of available surveillance data (for more information about the selection of epochs, see the Supplementary Appendix). For each epoch, we included participants 60 years of age or older who were free of dementia at the start of that epoch. Among participants with incident dementia during the epoch, follow-up time was measured in years from the baseline examination to the diagnosis of dementia. Data for participants in whom dementia did not develop were censored at the last date on which they were known not to have dementia, at the date of death, or up to 5 years after the baseline assessment (a complete observation period). Participants who did not have dementia at the end of an epoch could contribute information to a subsequent epoch.
In our primary analysis, we used Cox proportional-hazards models, adjusted for age at entry and sex, to compare the incidence of dementia across the four epochs. For each epoch, we report the 5-year cumulative hazard rates, which represent the cumulative incidence of dementia per 100 persons over a period of 5 years, and we report hazard ratios, which represent the incidence of dementia during each epoch relative to the incidence during the first epoch. We performed separate analyses for overall dementia, Alzheimer’s disease, and vascular dementia. We used robust sandwich estimators to account for the inclusion of individual participants in more than one epoch.25 We estimated linear trends, which represent the decline per decade in the 5-year incidence of dementia, using the elapsed mean time (in decades) between the first epoch and each consecutive epoch.
In secondary analyses, we examined possible interactions between epoch and age, sex, educational level, and APOE ε4 status. We also examined the effects of additional adjustments for educational level and individual vascular risk factors that were present at baseline or at midlife (i.e., 55±5 years of age), as well as the combined effect of vascular risk factors, as assessed by calculation of Framingham Stroke Risk Profile scores (for more information about the Framingham Stroke Risk Profile, see the Supplementary Appendix).26 Finally, we explored temporal trends in the effects of stroke and three common clinical cardiovascular diseases (atrial fibrillation, coronary heart disease, and heart failure) on the risk of dementia. Statistical analyses were performed with the use of SAS software, version 9.4 (SAS Institute).
RESULTS
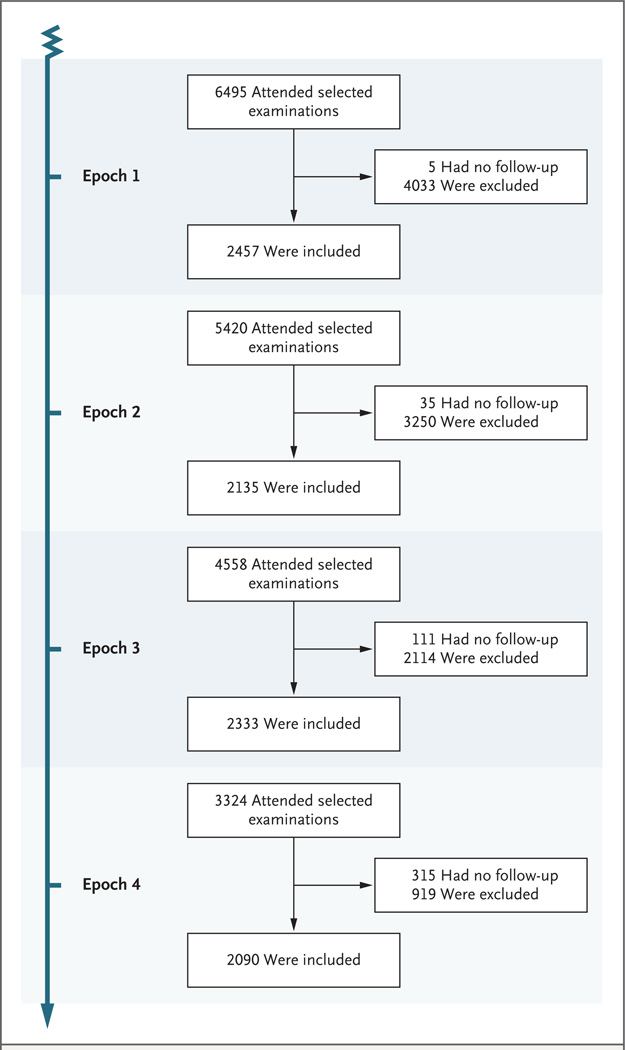
The analyses included 5205 individual participants who collectively contributed data for a total of 9015 observation periods (40,192 person-years). Across the four epochs, the proportion of living participants who attended the baseline examination remained constant at 75 to 78%, and the mean baseline MMSE scores did not differ among epochs (see Table S2 in the Supplementary Appendix). More than 2000 participants contributed data for each epoch (Fig. 1), and the participants’ ages ranged from 60 to 101 years (Table 1). Most health indicators were similar among participants in the original cohort and those in the offspring cohort. Over the three decades, we observed a trend toward higher educational level and a parallel trend toward a lower prevalence of most vascular risk factors, with the exception of obesity and diabetes, both of which were associated with increasing trends over time. We also observed trends toward a lower prevalence of stroke and other cardiovascular diseases. A deeper exploration revealed that improvements in cardiovascular health were seen only in the cohort of participants who had a high school diploma (see Tables S8 and S9 in the Supplementary Appendix).
Figure 1. Study Samples for the First, Second, Third, and Fourth Epochs.
The baseline examination period was between 1977 and 1983 for the first epoch, between 1986 and 1991 for the second epoch, between 1992 and 1998 for the third epoch, and between 2004 and 2008 for the fourth epoch. Participants who had no follow-up are those for whom we do not have verified information about cognitive status during the specified 5-year period; however, these participants were not lost to follow-up, and information about them may be available from later examinations or from additional sources. Participants were excluded from the analyses if they were younger than 60 years of age, did not attend examinations in which involvement in an inception cohort was established, or had preexisting dementia (for further details, see Fig. S1 in the Supplementary Appendix, available at NEJM.org).
Table 1.
Baseline Characteristics.*
| Characteristic | Epoch 1 (N = 2457) |
Epoch 2 (N = 2135) |
Epoch 3 (N = 2333) |
Epoch 4 (N = 2090) |
P Value for Trend |
|---|---|---|---|---|---|
| Age at entry (yr) | <0.001 | ||||
| Mean | 69±7 | 72±7 | 72±8 | 72±9 | |
| Range | 60–89 | 60–96 | 60–101 | 60–101 | |
| Female sex (%) | 59 | 57 | 57 | 56 | 0.01 (age-adjusted, <0.001) |
| Educational level (%) | <0.001 | ||||
| No high school diploma | 36 | 24 | 15 | 5 | |
| High school diploma | 32 | 37 | 37 | 32 | |
| Some years of college | 19 | 21 | 24 | 29 | |
| College degree | 13 | 17 | 24 | 34 | |
| Positive for at least one APOE ε4 allele (%)† | 22 | 21 | 21 | 21 | 0.47 (age-adjusted, 0.98) |
| Mean systolic blood pressure (mm Hg) | 137±19 | 143±22 | 138±20 | 131±18 | <0.001 |
| Mean diastolic blood pressure (mm Hg) | 76±10 | 77±11 | 73±10 | 72±10 | <0.001 |
| Use of antihypertensive medication (%) | 33 | 43 | 44 | 62 | <0.001 |
| Smoking (%) | 20 | 14 | 9 | 6 | <0.001 |
| Mean HDL cholesterol (mg/dl) | 50±16 | 49±15 | 50±16 | 57±18 | <0.001 |
| Use of lipid-lowering agents (%) | NA | NA | 12 | 43 | <0.001 |
| Mean body-mass index‡ | 26±4 | 27±5 | 27±5 | 28±5 | <0.001 |
| Type 2 diabetes (%) | 10 | 11 | 15 | 17 | <0.001 |
| Cardiovascular disease (%) | 23 | 26 | 25 | 22 | 0.52 (age-adjusted, <0.001) |
| Stroke (%) | 3.6 | 3.3 | 3.8 | 3.1 | 0.51 (age-adjusted, 0.02) |
Plus–minus values are means ±SD. The baseline examination period was between 1977 and 1983 for the first epoch, between 1986 and 1991 for the second epoch, between 1992 and 1998 for the third epoch, and between 2004 and 2008 for the fourth epoch. APOE denotes apolipoprotein E, HDL high-density lipoprotein, and NA not available.
Data were available for only a subgroup of participants with genotypic information, which included 1354 participants during the first epoch, 1989 during the second epoch, 2279 during the third epoch, and 2036 during the fourth epoch. The percentages are based on these data.
The body-mass index is the weight in kilograms divided by the square of the height in meters.
We observed 371 cases of dementia. There was a trend toward an increasing mean age at diagnosis, from 80 years during the first epoch to 85 years during the fourth epoch (P<0.001 for trend). The 5-year age- and sex-adjusted cumulative hazard rates for dementia declined over time; the rates were 3.6 (95% confidence interval [CI] 2.9 to 4.4) per 100 persons during the first epoch, 2.8 (95% CI, 2.2 to 3.5) per 100 persons during the second epoch, 2.2 (95% CI, 1.8 to 2.8) per 100 persons during the third epoch, and 2.0 (95% CI, 1.5 to 2.6) per 100 persons during the fourth epoch (Table 2). Relative to the incidence during the first epoch, the incidence of dementia declined by 22% during the second epoch (P = 0.09), by 38% during the third epoch (P = 0.001), and by 44% during the fourth epoch (P<0.001). On average, since 1977, there has been a decline in the incidence of dementia of 20% per decade (hazard ratio, 0.80; 95% CI, 0.72 to 0.90). The decline in the incidence of Alzheimer’s disease was not significant (P = 0.052 for trend), whereas the decline in the incidence of vascular dementia appeared to be more rapid than that of Alzheimer’s disease (P = 0.004 for trend), although analyses of the dementia subtypes are based on smaller numbers than are the analyses for overall dementia. There was no evidence to suggest that the interaction between epoch and age, sex, or APOE ε4 status had an effect on temporal trends in the incidence of dementia (P>0.10 for all comparisons), but the interaction between epoch and educational level had a significant effect (P = 0.03). Stratified analyses showed that the decline in the incidence of dementia was limited to the cohort of persons who had a high school diploma, with an average decline in risk of 23% per decade (hazard ratio, 0.77; 95% CI, 0.67 to 0.88); there was no decline in the cohort of persons who did not have a high school diploma (Table 3).
Table 2.
Temporal Trends in the Incidence of Dementia.*
| Subtype | No. of Cases |
Total No. of Observation Periods |
5-Yr Cumulative Hazard Rate (95% CI)† | 5-Yr Hazard Ratio (95% CI)‡ | P Value for Trend |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Epoch 1 | Epoch 2 | Epoch 3 | Epoch 4 | Epoch 2 | Epoch 3 | Epoch 4 | Trend§ | ||||
| Overall dementia | 371 | 9015 | 3.6 (2.9–4.4) |
2.8 (2.2–3.5) |
2.2 (1.8–2.8) |
2.0 (1.5–2.6) |
0.78 (0.59–1.04) |
0.62 (0.47–0.83) |
0.56 (0.41–0.77) |
0.80 (0.72–0.90) |
<0.001 |
| Alzheimer’s disease | 264 | 9015 | 2.0 (1.5–2.6) |
2.0 (1.5–2.6) |
1.7 (1.3–2.3) |
1.4 (1.0–1.9) |
1.00 (0.70–1.43) |
0.88 (0.62–1.25) |
0.70 (0.48–1.03) |
0.88 (0.77–1.00) |
0.052 |
| Vascular dementia | 84 | 9014 | 0.8 (0.6–1.3) |
0.8 (0.5–1.2) |
0.4 (0.2–0.7) |
0.4 (0.2–0.7) |
0.89 (0.51–1.56) |
0.46 (0.25–0.86) |
0.45 (0.23–0.87) |
0.71 (0.56–0.90) |
0.004 |
The baseline examination period was between 1977 and 1983 for the first epoch, between 1986 and 1991 for the second epoch, between 1992 and 1998 for the third epoch, and between 2004 and 2008 for the fourth epoch.
The 5-year cumulative hazard rates (the cumulative incidence of dementia per 100 persons over a period of 5 years) are adjusted for age and sex.
The 5-year hazard ratios (the incidence of dementia during each epoch relative to the incidence during the first epoch) are adjusted for age and sex.
We estimated linear trends (the decline per decade in the 5-year incidence of dementia) using the elapsed mean time (in decades) between the first epoch and each consecutive epoch.
Table 3.
Temporal Trends in the Incidence of Dementia, Stratified by Age, Sex, Educational Level, and Apolipoprotein E ε4 Status.*
| Variable | No. of Cases of Dementia |
Total No. of Observation Periods |
P Value for Interaction |
5-Yr Hazard Ratio (95% CI)† |
P Value for Trend |
|||
|---|---|---|---|---|---|---|---|---|
| Epoch 2 | Epoch 3 | Epoch 4 | Trend‡ | |||||
| Age at entry (yr) | 0.82 | |||||||
| 60–69 | 42 | 4418 | 0.43 (0.18–1.00) |
0.36 (0.15–0.89) |
0.38 (0.15–0.93) |
0.65 (0.47–0.89) |
0.008 | |
| 70–79 | 133 | 3229 | 0.91 (0.59–1.42) |
0.67 (0.42–1.07) |
0.64 (0.36–1.11) |
0.83 (0.68–1.00) |
0.047 | |
| ≥80 | 196 | 1368 | 0.86 (0.56–1.33) |
0.72 (0.48–1.09) |
0.68 (0.44–1.06) |
0.86 (0.74–1.01) |
0.06 | |
| Sex | 0.27 | |||||||
| Female | 234 | 5173 | 0.70 (0.50–1.00) |
0.52 (0.36–0.74) |
0.53 (0.36–0.78) |
0.77 (0.67–0.89) |
<0.001 | |
| Male | 137 | 3842 | 0.96 (0.59–1.57) |
0.89 (0.55–1.43) |
0.64 (0.38–1.08) |
0.85 (0.71–1.02) |
0.08 | |
| Educational level | 0.031 | |||||||
| No high school diploma | 130 | 1831 | 1.46 (0.94–2.26) |
0.97 (0.58–1.61) |
1.66 (0.87–3.15) |
1.11 (0.89–1.39) |
0.34 | |
| High school diploma | 228 | 6948 | 0.54 (0.36–0.81) |
0.55 (0.38–0.79) |
0.46 (0.31–0.67) |
0.77 (0.67–0.88) |
<0.001 | |
| APOE ε4 status§ | 0.15 | |||||||
| Any genotypic information | 246 | 6304 | 0.96 (0.70–1.30) |
0.83 (0.60–1.16) |
0.89 (0.74–1.08) |
0.25 | ||
| Negative for APOE ε4 | 169 | 5000 | 0.95 (0.65–1.37) |
0.75 (0.50–1.13) |
0.84 (0.66–1.06) |
0.14 | ||
| Positive for at least one APOE ε4 allele |
77 | 1304 | 1.01 (0.58–1.75) |
1.09 (0.61–1.93) |
1.05 (0.75–1.47) |
0.76 | ||
The baseline examination period was between 1977 and 1983 for the first epoch, between 1986 and 1991 for the second epoch, between 1992 and 1998 for the third epoch, and between 2004 and 2008 for the fourth epoch.
The 5-year hazard ratios (the incidence of dementia during each epoch relative to the incidence during the first epoch) are adjusted for age and sex, except for those stratified by sex (which were adjusted only for age at entry) and those stratified by age (which were adjusted only for sex).
We estimated linear trends (the decline per decade in the 5-year incidence of dementia) using the elapsed mean time (in decades) between the first epoch and each consecutive epoch.
Data were available for only a subgroup of participants with genotypic information. Data for APOE ε4 genotyping were limited during the first epoch; therefore, the hazard ratios for the third and fourth epochs were calculated relative to the data obtained during the second epoch.
Adjustment for vascular risk factors that were present at baseline or midlife did not significantly modify our results (Table 4, and see Tables S10 and S11 in the Supplementary Appendix). Adjustment for preexisting and incident stroke and for other cardiovascular diseases also led to nearly unchanged estimates. This was true even though the risk of dementia after a stroke decreased over time. During the first epoch, the risk of dementia among persons who had had a stroke was almost 9 times as high as the risk among those who had not had a stroke, but during the fourth epoch, the risk was less than 2 times as high (P = 0.06 for interaction) (see Table S12 in the Supplementary Appendix). Whereas the effect of physiological measures (e.g., a rise in blood pressure of 10 mm Hg) on the risk of dementia was identical across epochs, the adverse effect of heart failure and atrial fibrillation was less during the third epoch than during the first (P<0.10 for interaction), but this effect did not completely explain the observed declines in the incidence of dementia.
Table 4.
Temporal Trends in the Incidence of Dementia, Adjusted for Educational Level, Vascular Risk Factors at Midlife, and Cardiovascular Disease.*
| Variable | No. of Cases of Dementia |
Total No. of Observation Periods |
5-Yr Hazard Ratio (95% CI)† |
P Value for Trend |
|||
|---|---|---|---|---|---|---|---|
| Epoch 2 | Epoch 3 | Epoch 4 | Trend‡ | ||||
| High school diploma | 358 | 8778 | 0.82 (0.61–1.10) |
0.68 (0.50–0.91) |
0.65 (0.47–0.91) |
0.85 (0.75–0.95) |
0.005 |
| Increase in systolic blood pressure at midlife |
361 | 8837 | 0.76 (0.56–1.01) |
0.61 (0.46–0.81) |
0.54 (0.40–0.75) |
0.79 (0.71–0.89) |
<0.001 |
| Increase in body-mass index at midlife |
352 | 8658 | 0.78 (0.59–1.05) |
0.62 (0.47–0.83) |
0.56 (0.41–0.76) |
0.80 (0.71–0.89) |
<0.001 |
| Type 2 diabetes at midlife | 284 | 7418 | 0.76 (0.55–1.06) |
0.55 (0.39–0.76) |
0.50 (0.35–0.71) |
0.76 (0.67–0.86) |
<0.001 |
| Preexisting and incident stroke | 371 | 9015 | 0.78 (0.59–1.04) |
0.62 (0.47–0.82) |
0.58 (0.42–0.78) |
0.81 (0.72–0.90) |
<0.001 |
| Preexisting cardiovascular disease | 371 | 9015 | 0.78 (0.59–1.04) |
0.62 (0.47–0.82) |
0.57 (0.41–0.77) |
0.80 (0.72–0.90) |
<0.001 |
| Preexisting atrial fibrillation | 371 | 9015 | 0.78 (0.59–1.04) |
0.61 (0.46–0.81) |
0.55 (0.40–0.75) |
0.79 (0.71–0.89) |
<0.001 |
The baseline examination period was between 1977 and 1983 for the first epoch, between 1986 and 1991 for the second epoch, between 1992 and 1998 for the third epoch, and between 2004 and 2008 for the fourth epoch.
The hazard ratios in each row are adjusted for the variable shown in the first column of that row. In addition, the 5-year hazard ratios (the incidence of dementia during each epoch relative to the incidence during the first epoch) are adjusted for age and sex.
We estimated linear trends (the decline per decade in the 5-year incidence of dementia) using the elapsed mean time (in decades) between the first epoch and each consecutive epoch.
DISCUSSION
Results from the Framingham Heart Study showed a progressive decline in the incidence of dementia over three decades. This temporal trend and a parallel improvement in cardiovascular health over time were both observed only in the cohort of persons who had at least a high school diploma. Rising educational levels might have contributed to the 5-year delay we observed in the mean age at onset of clinical dementia. However, the proportion of participants who did not have a high school diploma was low during the last two epochs, thus limiting a deeper investigation of trends in the incidence of dementia in this subgroup.
Few studies can accurately track the incidence of dementia over time, and our study provides robust evidence that indicates a declining trend. Data from Rochester, Minnesota, showed a 30% decline in the age-adjusted incidence of dementia during the second decade they studied (1985–1994), but no significant trend was observed when the entire study period (1975–1994) was considered.7 Moreover, in that study, estimates of the incidence of dementia could have been affected by changes in clinical practice and diagnostic criteria for dementia over time, since ascertainment of events was obtained by linkage with medical records rather than with the use of standardized protocols. Although we also use data from medical records in the Framingham Heart Study, we supplement these data with direct assessments of the participants and their families. The Rotterdam Study suggested a 25% reduction in the incidence of dementia over a 10-year period through a comparison of the incidence rates in 1990 and 2000,6 but the results did not reach significance. Studies from the United States,7 England,8 and Stockholm9 have indirectly suggested declines in the incidence of dementia on the basis of repeated prevalence estimates drawn from survey data. In these studies, a true decline in the incidence of dementia cannot be distinguished from a faster increase in life expectancy among persons who do not have dementia than among those who have dementia.
In parallel with the trend toward a lower incidence of dementia, participants in the Framingham Heart Study also had improvements in most indicators of cardiovascular health, with the exception of a trend toward increasing prevalences of diabetes and obesity; this trend is consistent with national and global statistics.27 Although the age-adjusted prevalence of some vascular risk factors has decreased, the effect of specific vascular risk factors (e.g., elevated blood pressure) on the risk of dementia appears to have remained constant across epochs. However, we observed a decreasing effect of cardiovascular events and an increasing benefit of the use of antihypertensive medications on the subsequent risk of dementia during successive epochs. These findings suggest that earlier diagnosis and more effective treatment of stroke and heart disease might have contributed to a lower incidence of dementia, particularly vascular dementia, during more recent epochs. This benefit seems to be more pronounced among persons who have a high school diploma, a finding that is consistent with our observation that improvement in cardiovascular health was seen only among persons with at least a high school education.
Since vascular risk factors increase the risk of stroke and, in turn, a history of stroke increases the risk of cognitive decline and dementia, we examined the effect of adjustment for incident stroke on the risk of dementia; however, such an adjustment did not appreciably diminish the observed trends. Similarly, adjustment for the Framingham Stroke Risk Profile score and its components (including systolic blood pressure, use of antihypertensive medications, diabetes status, smoking status, atrial fibrillation, and clinical cardiovascular events) did not explain the decline, regardless of whether the data were obtained at baseline or at midlife. Our observations do not rule out a role for vascular risk factors in explaining the observed trends, although they emphasize the need to simultaneously search for additional explanations.
In addition to changes in vascular risk factors, temporal trends in the prevalence of neurodegenerative processes have also been documented. A recent study from Switzerland that evaluated 1599 specimens of brain tissue obtained from autopsies performed over the course of three decades (1972–2006) from persons who were 65 years of age or older at death suggests a decline in the age-adjusted burden of amyloid deposition.28 This intriguing trend might be caused by changes in education and vascular risk factors that are similar to those we observed in the Framingham Heart Study or could be due to factors we could not consider, such as changes in diet, physical activity, exposures to environmental toxins, or other unknown factors. The Framingham Heart Study does not have brain autopsy data from before 1995, and thus we are unable to explore the contribution of trends in amyloid burden to the observed risk of dementia across epochs.
A strength of the Framingham Heart Study is the long period of surveillance; dementia events have been tracked since 1975 and continue to be tracked to date. This permitted the assessment of temporal trends over three decades in a single cohort with carefully ascertained longitudinal data on various vascular risk factors. The Framingham Heart Study–based risk estimates, which are intermediate between the highest29 and lowest30 national estimates, are considered to be reliable and have been used by the Alzheimer’s Association to educate the public on lifetime risks.14,31 Although our diagnostic criteria for dementia have evolved over time, we were able to rereview all available records to retrospectively apply consistent diagnostic criteria to the entire 30-year period, thus reducing the risk of bias due to differences in diagnostic thresholds. Our tracking system for dementia is as consistent and accurate as possible in the setting of a longitudinal study, but we acknowledge that the awareness of dementia as a diagnostic entity has grown over the past 15 years. However, any resulting bias is likely to increase sensitivity for incident dementia in more recent epochs and should create a bias against finding a declining trend. Although the increase in educational level could have reduced the sensitivity of our multistep protocol for dementia screening, we performed complete neuropsychological assessments in a large number of participants who met the MMSE screening criteria and found no evidence that our MMSE cutoffs, which were adjusted for educational level, were insensitive among participants with high levels of education (see Tables S13 and S14 in the Supplementary Appendix).
One of the limitations of the Framingham Heart Study is that the participants are overwhelmingly of European ancestry; therefore, our findings would need to be replicated in groups that include a larger number of participants of other races and ethnic backgrounds. Furthermore, data were not available to examine the effects of some putative risk factors for dementia, such as diet and physical activity, as possible explanations for the observed temporal trends. Also, we were unable to consider the burden of subclinical vascular brain injury as a possible explanation for the observed trends, since the participants have undergone magnetic resonance imaging of the head only since 1999.
Despite our observation of a declining trend in the age-specific incidence of dementia and the possible stabilization of dementia occurrence in Western Europe,32 the worldwide burden of dementia will continue to increase rapidly as the average life expectancy increases. This is especially true for the most economically vulnerable persons, the most elderly persons in high-income countries,33 and persons in low-to-middle-income countries,34,35 where the average life expectancy and the burden of vascular risk factors are increasing most rapidly.
In conclusion, although projections suggest an exploding burden of dementia over the next four decades owing to an increasing number of older persons at risk,4,36 primary and secondary prevention might be key to diminishing the magnitude of this expected increase.37 Our study offers cautious hope that some cases of dementia might be preventable or at least delayed. However, it also emphasizes our incomplete understanding of the observed temporal trend and the need for further exploration of factors that contribute to this decline in order to better understand and possibly accelerate this beneficial trend.
Supplementary Material
Acknowledgments
Supported by the National Heart, Lung, and Blood Institute Framingham Heart Study (contract no. N01-HC-25195 and no. HHSN268201500001I) and by grants from the National Institute on Aging (AG08122 and AG033193) and the National Institute of Neurological Disorders and Stroke (NS017950).
We thank the study participants, as well as the study team (especially the investigators and staff of the neurology team) for their contributions to data collection over the past four decades.
Footnotes
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Aging, the National Institutes of Health, or the National Heart, Lung, and Blood Institute.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
REFERENCES
- 1.Sousa RM, Ferri CP, Acosta D, et al. Contribution of chronic diseases to disability in elderly people in countries with low and middle incomes: a 10/66 Dementia Research Group population-based survey. Lancet. 2009;374:1821–1830. doi: 10.1016/S0140-6736(09)61829-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sousa RM, Ferri CP, Acosta D, et al. The contribution of chronic diseases to the prevalence of dependence among older people in Latin America, China and India: a 10/66 Dementia Research Group population-based survey. BMC Geriatr. 2010;10:53. doi: 10.1186/1471-2318-10-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harwood RH, Sayer AA, Hirschfeld M. Current and future worldwide prevalence of dependency, its relationship to total population, and dependency ratios. Bull World Health Organ. 2004;82:251–258. [PMC free article] [PubMed] [Google Scholar]
- 4.Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement. 2013;9:63–75.e2. doi: 10.1016/j.jalz.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 5.Hurd MD, Martorell P, Delavande A, Mullen KJ, Langa KM. Monetary costs of dementia in the United States. N Engl J Med. 2013;368:1326–1334. doi: 10.1056/NEJMsa1204629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schrijvers EM, Verhaaren BF, Koudstaal PJ, Hofman A, Ikram MA, Breteler MM. Is dementia incidence declining? Trends in dementia incidence since 1990 in the Rotterdam Study. Neurology. 2012;78:1456–1463. doi: 10.1212/WNL.0b013e3182553be6. [DOI] [PubMed] [Google Scholar]
- 7.Rocca WA, Petersen RC, Knopman DS, et al. Trends in the incidence and prevalence of Alzheimer’s disease, dementia, and cognitive impairment in the United States. Alzheimers Dement. 2011;7:80–93. doi: 10.1016/j.jalz.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matthews FE, Arthur A, Barnes LE, et al. A two-decade comparison of prevalence of dementia in individuals aged 65 years and older from three geographical areas of England: results of the Cognitive Function and Ageing Study I and II. Lancet. 2013;382:1405–1412. doi: 10.1016/S0140-6736(13)61570-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qiu C, von Strauss E, Bäckman L, Winblad B, Fratiglioni L. Twenty-year changes in dementia occurrence suggest decreasing incidence in central Stockholm, Sweden. Neurology. 2013;80:1888–1894. doi: 10.1212/WNL.0b013e318292a2f9. [DOI] [PubMed] [Google Scholar]
- 10.Manton KC, Gu XL, Ukraintseva SV. Declining prevalence of dementia in the U.S. elderly population. Adv Gerontol. 2005;16:30–37. [PubMed] [Google Scholar]
- 11.Hebert LE, Bienias JL, Aggarwal NT, et al. Change in risk of Alzheimer disease over time. Neurology. 2010;75:786–791. doi: 10.1212/WNL.0b013e3181f0754f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dawber TR, Meadors GF, Moore FE., Jr Epidemiological approaches to heart disease: the Framingham Study. Am J Public Health Nations Health. 1951;41:279–281. doi: 10.2105/ajph.41.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families: the Framingham offspring study. Am J Epidemiol. 1979;110:281–290. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 14.Seshadri S, Wolf PA, Beiser A, et al. Lifetime risk of dementia and Alzheimer’s disease. The impact of mortality on risk estimates in the Framingham Study. Neurology. 1997;49:1498–1504. doi: 10.1212/wnl.49.6.1498. [DOI] [PubMed] [Google Scholar]
- 15.Seshadri S, Beiser A, Au R, et al. Operationalizing diagnostic criteria for Alzheimer’s disease and other age-related cognitive impairment-Part 2. Alzheimers Dement. 2011;7:35–52. doi: 10.1016/j.jalz.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farmer ME, White LR, Kittner SJ, et al. Neuropsychological test performance in Framingham: a descriptive study. Psychol Rep. 1987;60:1023–1040. doi: 10.1177/0033294187060003-201.1. [DOI] [PubMed] [Google Scholar]
- 17.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 18.Au R, Seshadri S, Wolf PA, et al. New norms for a new generation: cognitive performance in the Framingham offspring cohort. Exp Aging Res. 2004;30:333–358. doi: 10.1080/03610730490484380. [DOI] [PubMed] [Google Scholar]
- 19.Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment — beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256:240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- 20.Au R, Seshadri S, Knox K, et al. The Framingham Brain Donation Program: neuropathology along the cognitive continuum. Curr Alzheimer Res. 2012;9:673–686. doi: 10.2174/156720512801322609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diagnostic and statistical manual of mental disorders: DSM-IV. 4th. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 22.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 23.Bachman DL, Wolf PA, Linn R, et al. Prevalence of dementia and probable senile dementia of the Alzheimer type in the Framingham Study. Neurology. 1992;42:115–119. doi: 10.1212/wnl.42.1.115. [DOI] [PubMed] [Google Scholar]
- 24.Román GC, Tatemichi TK, Erkinjuntti T, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology. 1993;43:250–260. doi: 10.1212/wnl.43.2.250. [DOI] [PubMed] [Google Scholar]
- 25.Lee EW, Wei LJ, Amato DA, Leurgans S. Cox-type regression analysis for large numbers of small groups of correlated failure time observations. In: Klein JP, Goel PK, editors. Survival analysis: state of the art. Dordrecht, The Netherlands: Kluwer Academic; 1992. pp. 237–247. [Google Scholar]
- 26.Wolf PA, D’Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: a risk profile from the Framingham Study. Stroke. 1991;22:312–318. doi: 10.1161/01.str.22.3.312. [DOI] [PubMed] [Google Scholar]
- 27.Seidell JC. Obesity, insulin resistance and diabetes — a worldwide epidemic. Br J Nutr. 2000;83(Suppl 1):S5–S8. doi: 10.1017/s000711450000088x. [DOI] [PubMed] [Google Scholar]
- 28.Kövari E, Herrmann FR, Bouras C, Gold G. Amyloid deposition is decreasing in aging brains: an autopsy study of 1,599 older people. Neurology. 2014;82:326–331. doi: 10.1212/WNL.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 29.Evans DA, Bennett DA, Wilson RS, et al. Incidence of Alzheimer disease in a biracial urban community: relation to apolipoprotein E allele status. Arch Neurol. 2003;60:185–189. doi: 10.1001/archneur.60.2.185. [DOI] [PubMed] [Google Scholar]
- 30.Kawas C, Gray S, Brookmeyer R, Fozard J, Zonderman A. Age-specific incidence rates of Alzheimer’s disease: the Baltimore Longitudinal Study of Aging. Neurology. 2000;54:2072–2077. doi: 10.1212/wnl.54.11.2072. [DOI] [PubMed] [Google Scholar]
- 31.Seshadri S, Wolf PA. Lifetime risk of stroke and dementia: current concepts, and estimates from the Framingham Study. Lancet Neurol. 2007;6:1106–1114. doi: 10.1016/S1474-4422(07)70291-0. [DOI] [PubMed] [Google Scholar]
- 32.Wu YT, Fratiglioni L, Matthews FE, et al. Dementia in western Europe: epidemiological evidence and implications for policy making. Lancet Neurol. 2015;15:116–124. doi: 10.1016/S1474-4422(15)00092-7. [DOI] [PubMed] [Google Scholar]
- 33.Mathillas J, Lövheim H, Gustafson Y. Increasing prevalence of dementia among very old people. Age Ageing. 2011;40:243–249. doi: 10.1093/ageing/afq173. [DOI] [PubMed] [Google Scholar]
- 34.Chan KY, Wang W, Wu JJ, et al. Epidemiology of Alzheimer’s disease and other forms of dementia in China, 1990–2010: a systematic review and analysis. Lancet. 2013;381:2016–2023. doi: 10.1016/S0140-6736(13)60221-4. [DOI] [PubMed] [Google Scholar]
- 35.Wu YT, Brayne C, Matthews FE. Prevalence of dementia in East Asia: a synthetic review of time trends. Int J Geriatr Psychiatry. 2015;30:793–801. doi: 10.1002/gps.4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferri CP, Prince M, Brayne C, et al. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366:2112–2117. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011;10:819–828. doi: 10.1016/S1474-4422(11)70072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.