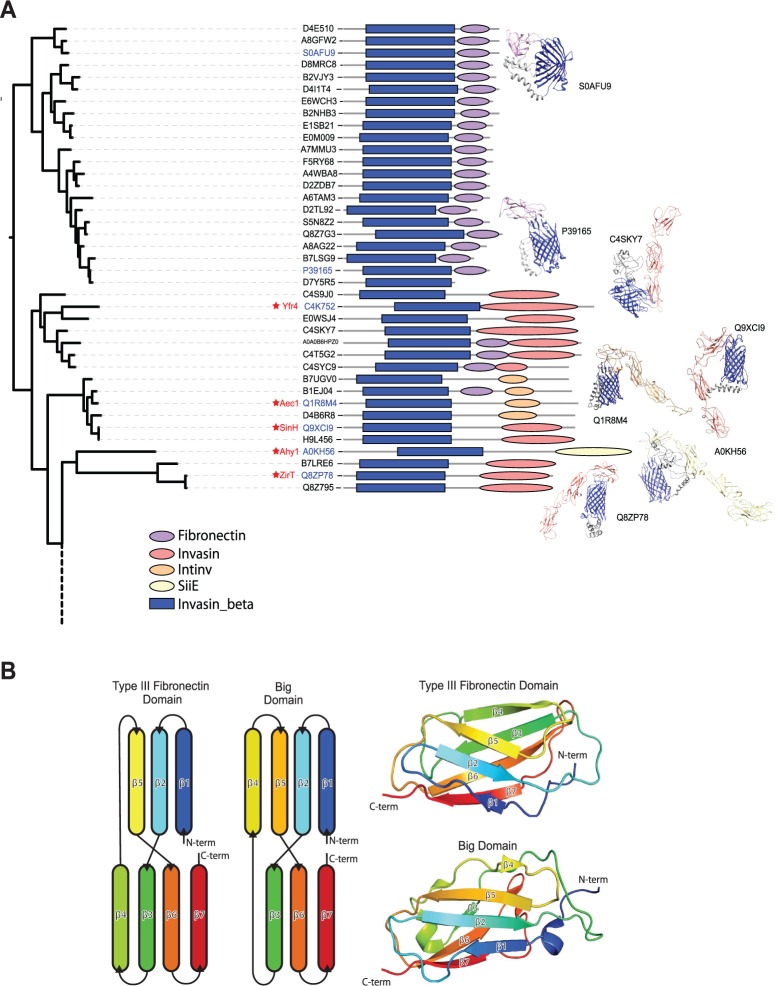
Fig. 7.—
Structural models of inverse autotransporter passenger domains. (A) The protein sequences without predicted Big domains (fig. 3) were subjected to structure prediction with Phyre2 (Kelley et al. 2015). Results (see supplementary table S7, Supplementary Material online) are illustrated as icons. Most of the sequences appear to conform to fibronectin-like domains (purple), others predict to fold into Big domain folds (orange/red). Structural models built with Phyre2 were edited in Chimera (Pettersen et al. 2004). (B) Structural comparison of the human type III fibronectin domain and a Big domain from FdeC shows that both domains possess an equivalent β-sandwich fold. A schematic representation of the β-sheets from each domain (left) illustrates the common β-sheet topology, while a cartoon representation (right) of Type III Fibronectin repeat 9 (pdb: 1FNF, residues 1327–1417) and FdeC region B Big1 domain (pdb: 4E9L, residues 678–793) shows the β-sandwich fold.