Figure 3.
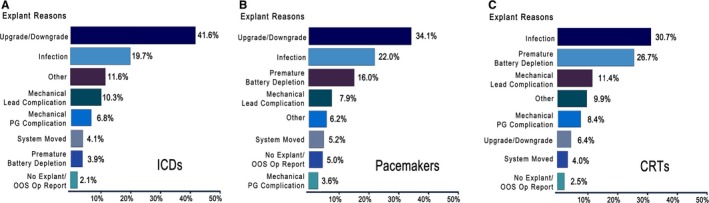
Reasons for early device revision, explantation, or replacement. During 2007–2013, 465 implantable cardioverter defibrillators (ICDs) were explanted, replaced, or revised after having been implanted for less than 36 months (A). 1018 Pacemakers were explanted, replaced, or revised after having been implanted for less than 60 months (B), and 182 cardiac resynchronization therapy devices were explanted, replaced, or revised after having been implanted for less than 36 months (C). Time frame for early explants was determined by electrophysiologist consultants. (1) “Mechanical Lead Complication” refers to devices functioning normally and with ample battery life replaced at the time of lead revision/replacement for a lead malfunction. (2) “Premature Battery Depletion” is classified as a form of device malfunction if the battery reaches elective replacement indicator or end of life and is replaced within 36/60 months of implant. (3) “Other” reasons include Pocket Erosion, Pocket Discomfort/Pain, Patient Requested Removal, and Patient Anatomy Issues. More than one reason may apply to a single revised device. Exclusions: elective replacement indicator, Heart Transplants, and Hospice Care.
