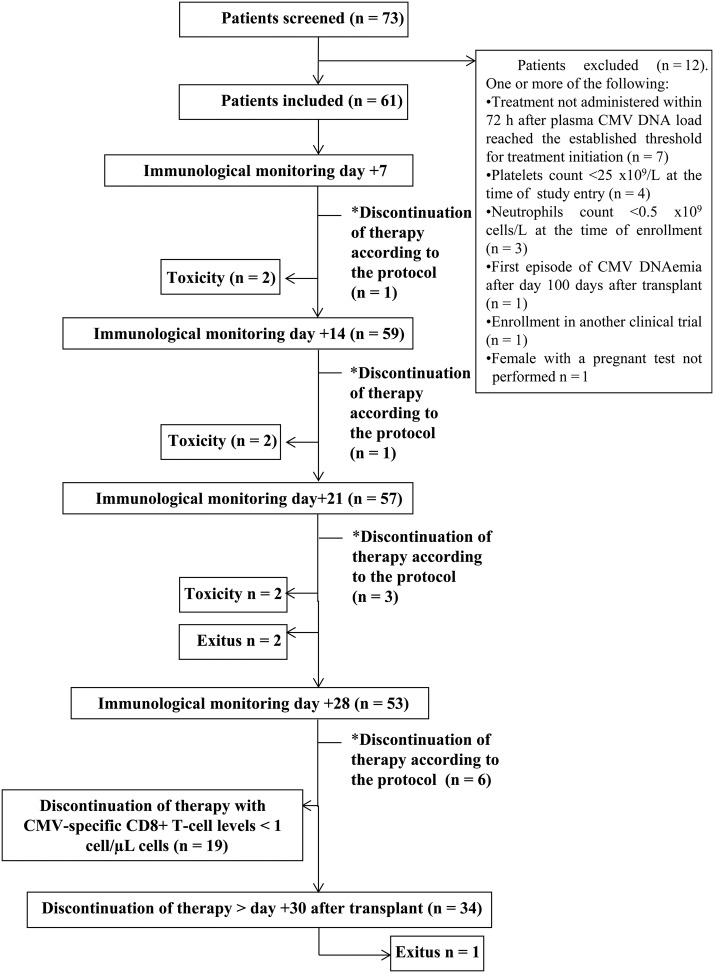
Figure 1.
Flow chart showing the screening, the enrollment status, and the developments in the study group (patients guided by the plasma cytomegalovirus [CMV] deoxyribonucleic acid [DNA] load and peripheral blood counts of pp65/IE1-interferon [IFN]-γ-producing CD8+ T cells). Anti-CMV therapy was scheduled to be withdrawn upon CMV DNAemia clearance and concurrent detection of pp65/IE-1-specific IFN-γ-producing CD8+ T cells at levels >1 cell/µL at any time within 1 month after therapy inception (*). Patients who left the study because of drug-related toxicity: 2 between days 7 and 14, 2 between days 14 and 21, and 2 between days 21 and 28. Two patients died between days 21 and 28. Antiviral treatment was interrupted within the study period (on day 28) with CMV-specific T-cell counts <1 cell/µL. Antiviral therapy was suspended in 23 patients after day 30 on the basis of virological criteria (exclusively).