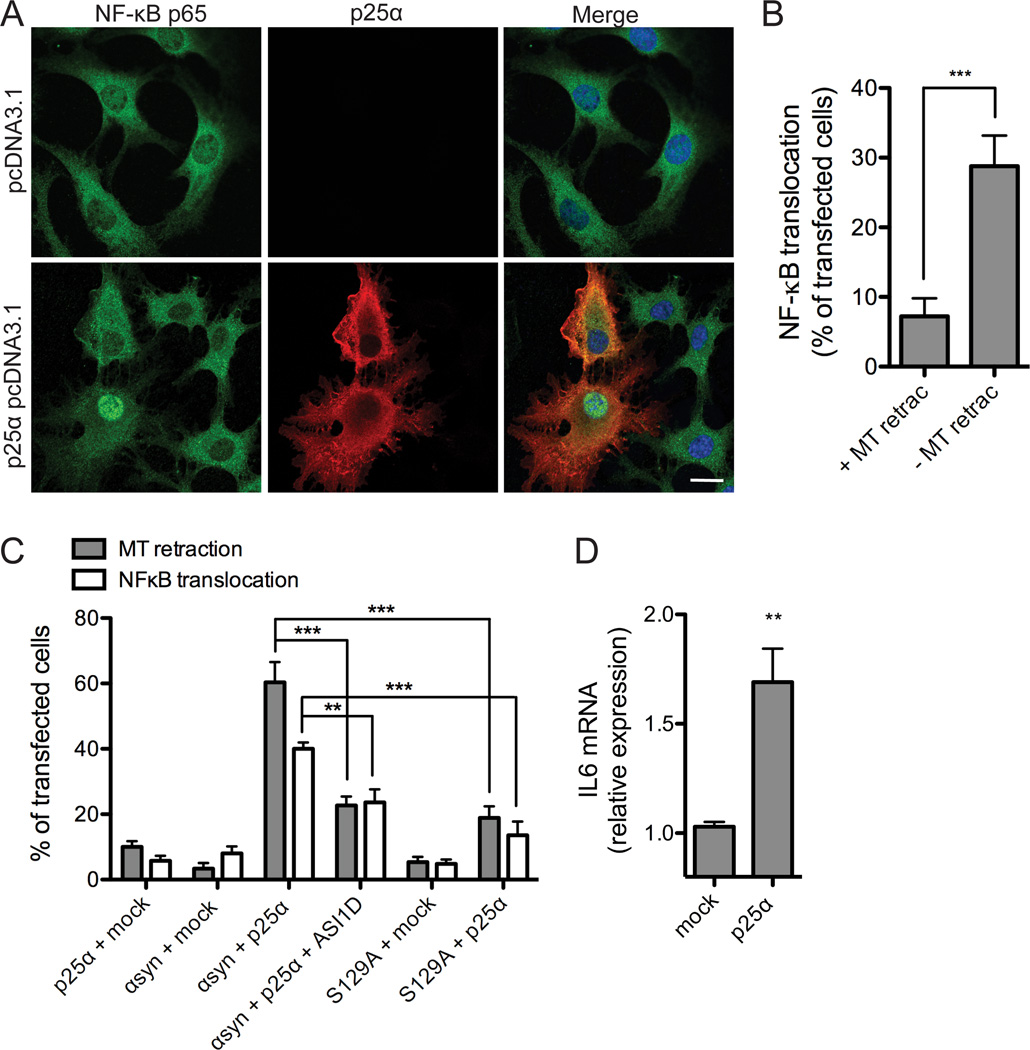
Figure 3. NF-κB activation accompanies α-synuclein dependent toxicity.
A, α-syn-expressing OLN-t40-AS cells were transiently transfected with an empty vector or p25α for 24 h and subjected to immunofluorescence microscopy using antibodies against NF-κB p65 (green) and p25α (red). Co-expression of α-syn and p25α causes NF-κB p65 translocation from the cytoplasm to the nucleus in a fraction of p25α expressing cells. Scale bar, 20 µm. B, NF-κB translocation was quantified in OLN-t40-AS cells transfected with p25α, which demonstrated normal flat (− MT retraction) or round (+ MT retraction) morphology. Bars represent mean ± S.D. from three independent experiments. The nuclear translocation of NF-κB p65 was significant higher in cells without MT retraction compared to “round” cells (with MT retraction) (***p < 0.001). C, Nuclear localization of NF-κB p65 was quantified in OLN-93 cells transiently expressing α-syn wt or α-syn S129A in combination with p25α or empty vector. Cells were treated with α-syn aggregation inhibitor peptide ASI1D when indicated. Bars represent mean ± S.D. from three independent experiments. NF-κB is translocated to the nucleus in response to coexpression of α-syn and p25α. Treatment with ASI1D peptide or coexpressing S129A with p25α significantly reduces the number of cells with MT retraction and nuclear NF-κB staining compared to coexpression of p25α and α-syn wt (**p < 0.01, ***p < 0.001). D, To investigate NF-κB responsive gene expression, IL6 mRNA levels were analyzed by real-time qPCR in OLN-t40-AS cells transiently transfected with p25α or empty vector for 24 h. Fold changes were determined from triplicate measurements and normalized to the NADH gene. Bars represent mean ± S.D. from three independent experiments. IL6 mRNA expression is increased 1.7-fold in cells coexpressing α-syn and p25α (p < 0.05).