Figure 5.
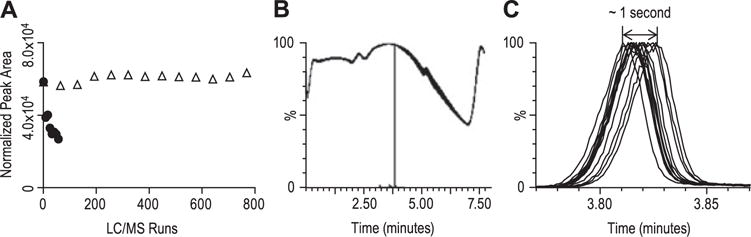
Reproducibility and robustness of the microfluidic device with tandem mass spectrometry. The peptide LFLEPTQADIALLK was synthesized with a 2H3-label on the second leucine from the N-terminus. Labeled peptide (10 nM) was added to rhesus plasma digests and used as quality controls, which was analyzed along with plasma samples from human subjects (800 LC/MS injections). (A) The variability of peptide peak area (M0: 786.5 → 1069.6): the plot with closed circles was from CaptiveSpray-TSQ Vantage, and open triangles was from the microfluidic device and the Xevo TQS. Using the CaptiveSpray source, the peak area decreased 50% within 50 LC/MS injections, and eventually the ion transfer tube became clogged. Using microfluidic device ionization source, the CV of the peak area was approximately 3.5% in the course of 800 LC/MS injections. (B) Overlay of backpressure traces of all QC samples, which was stable. (C) Overlay of selected ion chromatograms of all QC samples. The retention time shift was within 1 s.
