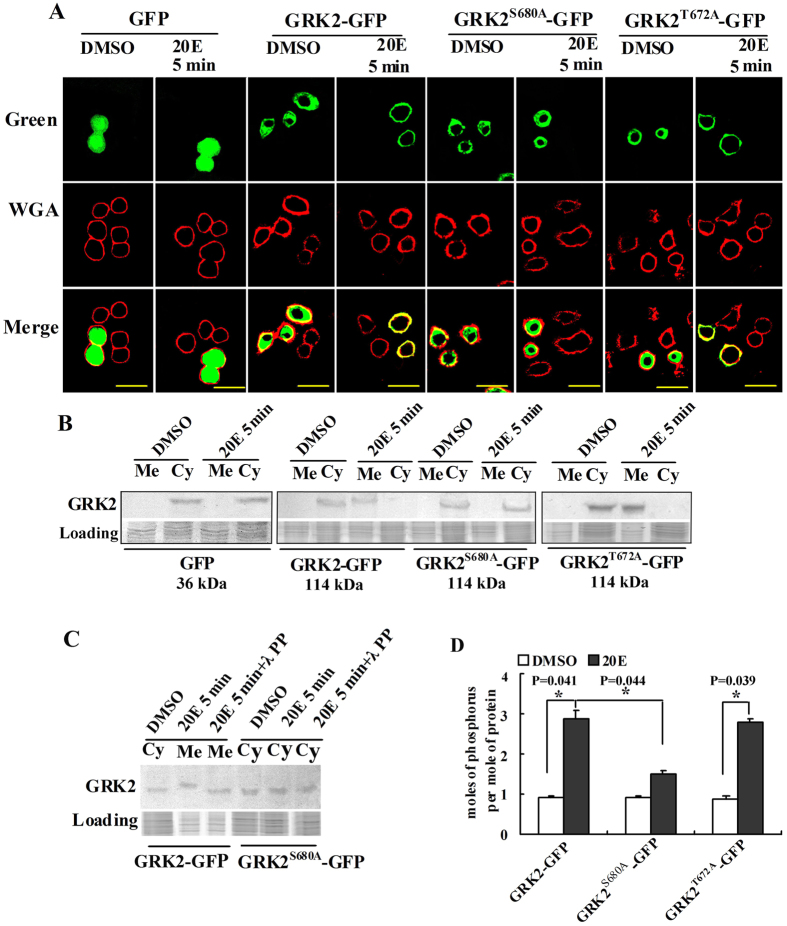
Figure 5. Serine 680 phosphorylation determines GRK2 membrane migration in response to induction by 20E.
(A) Green fluorescent protein (GFP), GRK2-GFP, GRK2T672A-GFP-His and GRK2S680A-GFP-His (green) were overexpressed by the plasmid GFP-pIEx-His in HaEpi cells after treatment with 20E (1 μM) or an equal volume of the DMSO control for 5 min and were viewed using a Zeiss LSM 700 laser confocal microscope. WGA (red) was used as a cell membrane marker. Merge is the overlapped green and red. The yellow bars denote 20 μm. (B) Western blot analysis for the overexpression and subcellular distribution of GRK2-GFP-His, GRK2T672A-GFP-His (114 kDa) and GRK2S680A-GFP-His (114 kDa) in HaEpi cells, which were detected using an antibody against GFP-tag. Cytoplasm (Cy) and membrane (Me) proteins were extracted after treatment with 20E (1 μM) or an equal volume of DMSO as a control for 5 min. SDS-PAGE with Coomassie Brilliant Blue staining was performed at the same time as loading of controls to normalize the protein amounts in the membrane and cytoplasm. Figure S10 are the full-length blots and gels data. The gels ran under the same experimental conditions. (C) Western blots to confirm phosphorylation of GRK2-GFP-His after 20E treatment by contrast with GRK2S680A-GFP-His using anti-GFP. SDS-PAGE gel with Coomassie Brilliant Blue staining was performed at the same time as loading of the control to normalize the protein amounts in the membrane (Me) and cytoplasm (Cy). λ PP: λ-protein-phosphatase (5 mM, 30 min at 30 °C). Figure S11 are the full-length blots and gels data. The gels ran under the same experimental conditions. (D) Numbers of moles of phosphorus per mole of GRK2-GFP-His, GRK2T672A-GFP-His and GRK2S680A-GFP-His were analyzed using a phosphoprotein phosphate estimation kit. The 20E concentration was 1 μM for 5 min. DMSO was used as the control. Asterisks indicate significant differences between the groups (p < 0.05) that were determined by Student’s t-test based on three independent experiments. The bars indicate the means + SD of three independent experiments.