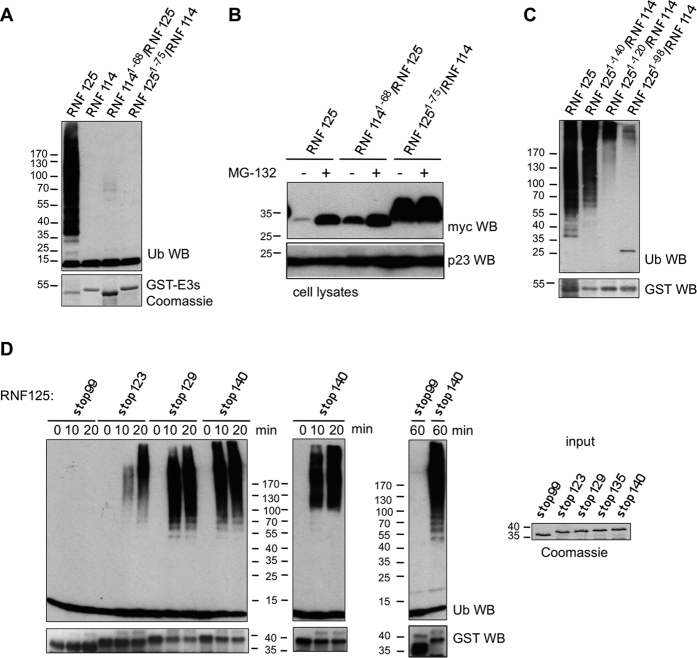
Figure 2. Ubiquitin ligase activity of RNF125/RNF114 chimeras and RNF125 stop mutants.
(A) In vitro ubiquitination reactions with GST fusion proteins of RNF125, RNF114 and the chimeras, for 60 min at 37 °C, were analyzed by Western blotting (WB) with anti-ubiquitin antibody (Ub). Coomassie staining shows the input levels of E3s. (B) HEK293T cells transfected with cDNAs for myc-tagged RNF125 or the RNF125/RNF114 chimeras, were either left untreated (-) or treated with MG-132 (20 μM) for 2 h. Cell lysates were analyzed by Western blotting for RNF125 levels with anti-myc. Blotting for the chaperone protein p23 was used as a loading control. (C) In vitro ubiquitination reactions with RNF125 and the indicated chimeras, performed as in panel A. Ubiquitination was detected with anti-ubiquitin (Ub) and input levels of the chimeras are shown by anti-GST Western blotting. The band around 25 kD in the RNF1251-98/RNF114 lane (Ub WB) most likely represents a multimer of ubiquitin. (D) In vitro ubiquitination reactions with GST fusion proteins of RNF125 stop mutants were stopped at 0, 10 and 20 min (two panels on left). The reactions were analyzed with anti-ubiquitin antibody (Ub) to detect ubiquitination, and subsequently with anti-GST (GST) to detect the fusion proteins. Reactions of RNF125stop99 and RNF125stop140 were also run for 60 min. To show relative input levels of E3s, the GST fusion proteins were also loaded separately and stained with Coomassie (panel on the right).