Fig. 2.
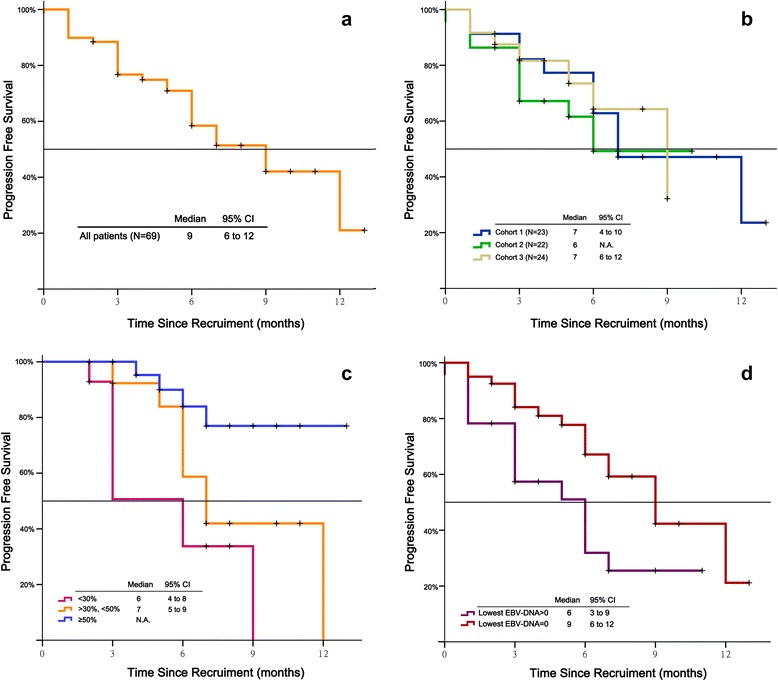
Kaplan-Meier analysis of median progression free survival in: a, all study patients; b, all 3 nab-paclitaxel dose cohorts; c, patients with ≤ 30 %, > 30 to < 50 %, and ≥ 50 % maximum decrease in the sum of the longest diameters of target lesions; and d, patients with complete EBV-DNA clearance at any time during treatment versus those with non-zero trough EBV-DNA. Cohort 1, cisplatin 75 mg/m2 day 1 + nab-paclitaxel 260 mg/m2 day 1 Q3W; Cohort 2, cisplatin 75 mg/m2 day 1 + nab-paclitaxel 140 mg/m2 day 1, 8 Q3W; Cohort 3, cisplatin 75 mg/m2 day 1 + nab-paclitaxel 100 mg/m2 day 1, 8, 15 Q3W. CI, confidence interval
