FIG 1.
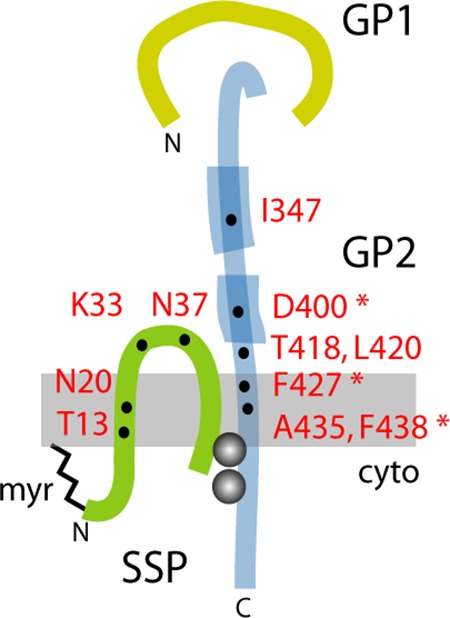
Model for the subunit organization of the tripartite GPC complex and location of resistance mutations. The SSP, GP1, and GP2 subunits are drawn schematically and are not to scale. Features include the myristate moiety at the N terminus of SSP (myr) (21), the binuclear zinc finger linking the penultimate cysteine-57 in SSP and the novel zinc-binding motif in the cytoplasmic tail of GP2 (gray balls) (54, 55), and the heptad-repeat regions in the ectodomain of GP2 that are diagnostic of class I viral fusion proteins (thickened lines) (25–28). Residues in JUNV GPC associated with resistance to or dependence on small-molecule fusion inhibitors are indicated (31, 32, 37, 40); asterisks denote mutations in GP2 that complement pH-dependent fusion defects engendered by mutations at K33 in SSP (24).
