Fig. 5.
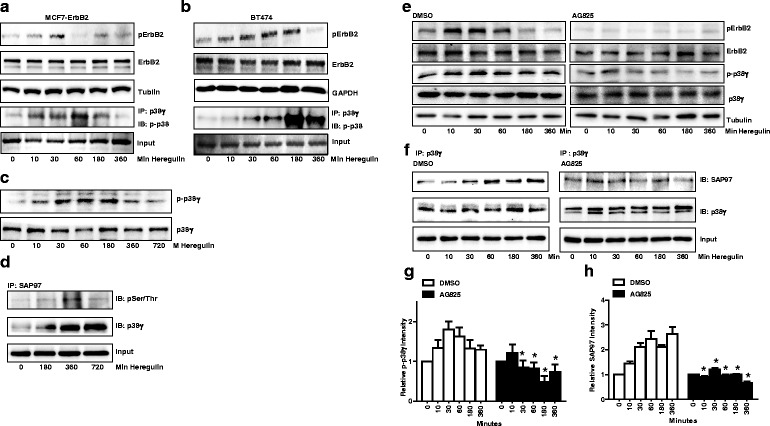
Effect of heregulin β1 on the activation of ErbB2, p38γ MAPK and SAP97. a MCF7-ErbB2 cells were treated with heregulin β1 (50 ng/ml) for the indicated times. The phosphorylation of ErbB2 (pErbB2) was determined by immunoblotting. The phosphorylation of p38γ was analyzed by immunoprecipitation. Cell lysates were first immunoprecipitated (IP) by an anti-p38γ antibody and then immunoblotted (IB) with an anti-pan-phosphorylated p38 MAPK. The experiment was replicated three times. b The heregulin β1-induced phosphorylation of ErbB2 and p38γ in BT474 cells was determined. The notations are the same as in panel (a). c The heregulin β1-induced phosphorylation of p38γ MAPK in MCF-ErbB2 cells was determined by immuoblotting using a phospho-specific antibody directed against p38γ MAPK (p-p38γ). d MCF7-ErbB2 cells were treated with heregulin β1 for indicated times. Equal amount of proteins were immunoprecipitated (IP) with an anti-SAP97 antibody, then immunoblotted (IB) using antibodies directed against phosphorylated serine/threonine or p38γ MAPK. e MCF7-ErbB2 cells were pretreated with DMSO or AG825 (50 μM) for 2 h followed by heregulin β1 treatment. The phosphorylation of ErbB2 and p38γ MAPK was determined by immunoblotting. f MCF7-ErbB2 cells were pretreated with DMSO or AG825 (50 μM) for 2 h followed by heregulin β1 treatment. The interaction of p38γ MAPK and SAP97 was determined by immunoprecipitation. Equal amount of protein were immunoprecipitated (IP) with an anti-p38γ antibody, then immnoblotted (IB) with an anti-SAP97 or anti-p38γ MAPK antibody. The experiment was replicated three times. g and h The relative levels of p-p38γ and SAP97 were quantified, normalized to the loading control, and then expressed relative to time 0 in either DMSO or AG825 groups. Each data point was the mean ± SEM of three independent experiments. *denotes significant difference from control groups (p < 0.05)
