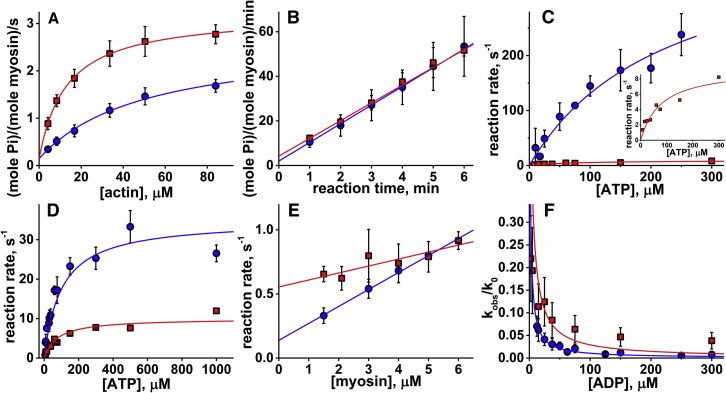
Figure 1.
(A) Steady-state, actin-activated myosin ATPase activity ([myosin] = 0.8 μM, [ATP] = 5 mM; circles, no Ficoll70; squares, with Ficoll70 in solution). Fit with a hyperbola, Vmax = 2.5 ± 0.1 s−1, no Ficoll70; and 3.1 ± 0.1 s−1 with Ficoll70. The data points are averages of N = 6 independent protein preparations. Uncertainties are the standard deviation here and throughout the text. (B) Basal myosin ATPase activity, [myosin] = 3.3 μM. Linear fit, N = 6, v = 0.140 ± 0.007 s−1, no Ficoll70, and 0.133 ± 0.002 s−1 with Ficoll70. (C) ATP-induced actomyosin dissociation. No Ficoll70, N = 6; with Ficoll70 in solution, N = 2. Fit with a hyperbola, Vmax = 431.6 ± 120.1 s−1, no Ficoll70; and 9.4 ± 1.4 s−1 with Ficoll70. Inset: data obtained with Ficoll70 in solution. (D) Kinetics of ATP binding and the recovery stroke upon rapid mixing of myosin and ATP; the observed rate constants are fitted with a hyperbola, Vmax = 34.9 ± 4.4 s−1, N = 4, no Ficoll70; and 10.0 ± 0.9 s−1, N = 2, with Ficoll70 in solution. (E) Kinetics of the weak-to-strong actomyosin binding transition, N = 4. The observed reaction rates are fitted by a straight line, and the second-order reaction rate constant is determined from the slope of the line. K8k′+4 = 0.13 ± 0.01 μM−1s−1, no Ficoll70; and 0.05 ± 0.01 μM−1s−1 with Ficoll70 in solution. (F) ATP-induced ADP dissociation from actomyosin ([actomyosin] = 0.5 μM, [ATP] = 25 μM; circles, no Ficoll70, N = 3; squares, with Ficoll70, N = 2). Lines: fit by the model of competitive inhibition with variable parameter K′5, K′5 = 1.07 ± 0.07 μM, no Ficoll70; and 1.58 ± 0.58 μM with Ficoll70. To see this figure in color, go online.