Abstract
Q fever is endemic in Australia, and during the period 2005–2013 our laboratory diagnosed 379 cases in New South Wales. To evaluate clinical symptoms, epidemiology, mode of diagnosis, antibody profiles, and treatment, a subset of 160 (42%) Q fever cases were analyzed in detail following the return of a questionnaire by the patient's doctor and from their laboratory reports. Overall, 82% patients were male and predominantly middle aged. The majority of patients (89%) had animal contact among which 63% were with cattle, 11% with sheep, and 7% with kangaroos. Clinical symptoms were nonspecific: myalgia (94%), fever (91%), headache (80%), acute fatigue (64%), and arthralgia (55%). Most cases (93%) were acute, and serology (immunofluorescence) was the main diagnostic modality. Positive real-time polymerase chain reaction results were useful in the diagnosis of both acute and chronic Q fever, as was the isolation of Coxiella burnetii in cell culture. Doxycycline was the antibiotic most commonly used.
BACKGROUND
Q fever was first recognized as a new disease in Australia in 19371 followed by detection of Coxiella burnetii in the United States.2 This infection has been recognized worldwide, with the exceptions of New Zealand3 and French Polynesia.4 It is a zoonosis with protean clinical manifestations and difficult to diagnose clinically, even with a definite history of animal contact. Laboratory investigations (mainly serology) are usually required to confirm the diagnosis of Q fever.
The purpose of the current study was to investigate a series of 379 symptomatic and laboratory confirmed cases in New South Wales (NSW), Australia, during 2005–2013. This study supplements earlier investigations into Q fever in other states of Australia: Powell5 and Derrick6 in Queensland, and Spellman7 and Buckley8 in Victoria.
METHODS
The Australian Rickettsial Reference Laboratory, a diagnostic and research laboratory, diagnoses Q fever following referral from doctors. All sera were collected from symptomatic patients seeking a diagnosis. Serology was undertaken by immunofluorescence, using both phase 2 and phase 1 C. burnetii antigens, and fluorescein-labeled antihuman serum (KPL, Gaithersburg, MD) used at 1/100 dilution to detect three immunoglobulin classes and total antibody to this bacterium. Patient sera were diluted to 1/25, and if positive, a doubling dilution series (1/25 to 1/3,200) of each serum was prepared.9 Coxiella burnetii Nine Mile strain phase 1 (NMI) was grown in wild-type mice following intraperitoneal inoculation and antigen harvesting from the spleen 7 days later. Coxiella burnetii, Nine Mile phase 2 /Clone 4 was grown in VERO cells, using RPMI-1640 medium (Life Technologies, Grand Island, NY), with 10% bovine fetal calf serum at 35°C in 5% CO2 for 2–3 weeks. Each antigen preparation was diluted to a suitable concentration (∼50–100 C. burnetii per high-power field) before spotting into glass slides and fixing with methanol.
Q fever DNA amplification was undertaken by two separate real-time polymerase chain reactions (qPCR) targeting two genes unique to C. burnetii, com1 and htpAB. A threshold cycle reading at or below 40, for both genes, was considered to be positive.10 Coxiella burnetii culture was undertaken by inoculating the patient serum into VERO cells and showing subsequent increases in qPCR positivity on a standard area of scraping of the cell monolayer.
In the case of negative serology, a request was made for follow-up serum to detect any subsequent seroconversion. In the case of a seropositive patient, a request was also made for a follow-up serum to look for any significant changes in antibody titers. A one page questionnaire was sent to all doctors whose patient had a confirmed case of Q fever. Of the 379 questionnaire sent, 160 (42%) were returned.
A case of Q fever was defined as: 1) culture of C. burnetii, 2) a positive qPCR assay from a patient sample (usually serum), or 3) positive serology. For diagnosing acute Q fever, the latter required a single serum with a positive phase 2 IgM (> 100) or positive phase 2 IgM plus positive phase 2 IgG (> 100) without positive phase 1 antibodies. For diagnosing chronic Q fever, a single serum with a positive phase 1 IgG (≥ 800) plus positive phase 1 IgA (≥ 200) was required. A second serum showing changes in antibody classes and titers over time gave a more convincing serological diagnosis. In all serologically defined cases, the clinical features were consistent with Q fever.
RESULTS
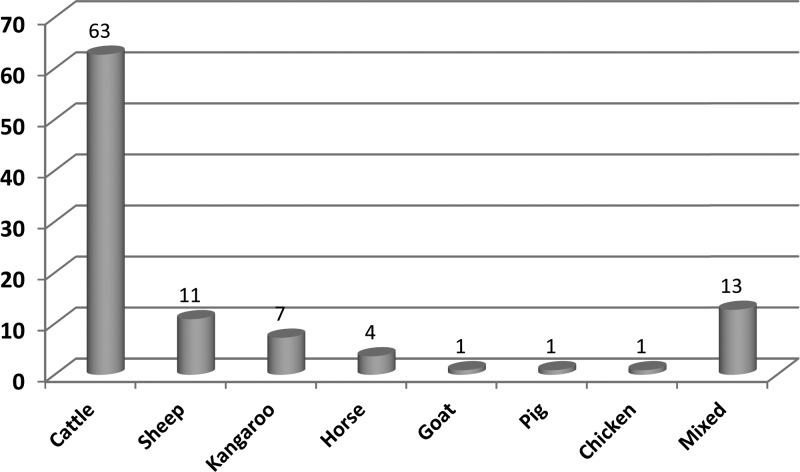
Of the 379 patients sequentially diagnosed with Q fever during 2005–2013, 82% were male. Of the 124 patients on whom the animal contact question was answered, 89% had animal contact, mostly with cattle (63%) although there were some cases that were associated with sheep, kangaroos, and other animals (Figure 1).
Figure 1.
Distribution of Q fever patients with specific animal contact (%) among patients in the animal contact group (n = 110).
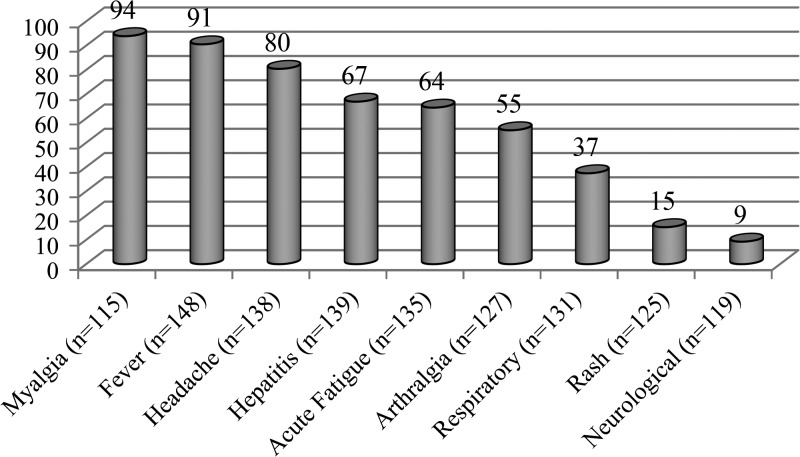
The main presenting clinical features were nonspecific (Figure 2) with nothing pointing to Q fever as the correct diagnosis. There were no pathognomonic symptoms or signs to assist the doctor in making the diagnosis of Q fever. In the 12 patients with chronic Q fever, two had endocarditis and one had osteomyelitis. Rash and neurological symptoms were absent from the 12 chronic Q fever cases. Overall, 67% of patients had hepatitis (based on abnormal liver function tests), 37% had respiratory symptoms (usually mild, e.g., cough), and 23% had both hepatitis and respiratory symptoms.
Figure 2.
Distribution (%) of clinical symptoms of Q fever patients.
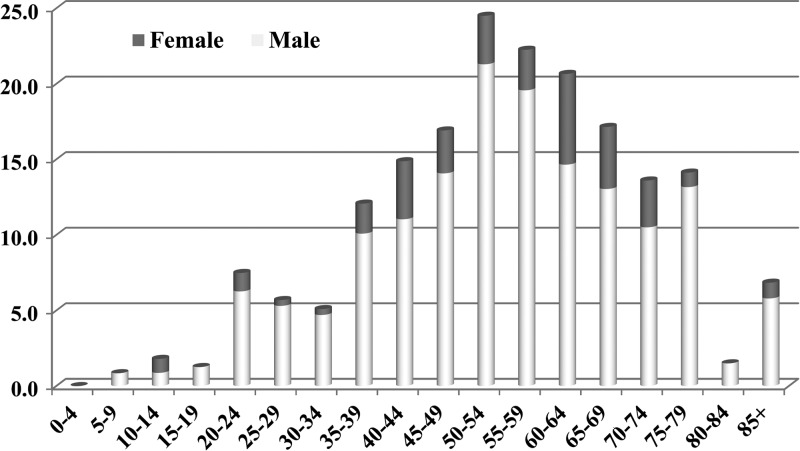
In prevalence by age, there were very few cases diagnosed in the 0- to 19-year-age group (Figure 3) but from that age onward the likelihood of infection increased in each age cohort up to 50–54 years, and then started to fall again. The age of major risk of Q fever in NSW was between 45 and 69 years.
Figure 3.
Age and sex distribution of 379 Q fever patients per 100,000 populations per annum.
Q fever diagnosis by immunofluorescence serology (Table 1 ) shows the total number of sera tested of the 160 patients, 51 of whom seroconverted. Among the 160 patients, only 40 cases were confirmed using a single serum. The remaining 120 cases provided a further one or more sera to confirm the diagnosis. Of the 51 patients who seroconverted, some seroconverted to only phase 2 antigen (N = 13), this being the first antibody to be produced in acute Q fever, but many (N = 38) seroconverted to the both phase 1 and phase 2 antigens. No patient seroconverted to only phase 1 antigen. Representative examples of antibody changes in the serum of acute and chronic patients (three patients each) are shown in Table 2. The order of antibody appearance following acute infection was: phase 2 IgM, phase 2 IgG, phase 1 IgM, phase 1 IgG, phase 2 IgA, and phase 1 IgA. The difference between patients with chronic Q fever and patients with acute Q fever were negative IgM (both phase 2 and phase 1), positive phase 1 IgA, and usually higher phase 1 IgG titers in chronic Q fever than in acute Q fever. Patient number 199 was initially diagnosed as acute Q fever with high phase 2 IgM titers but during 5 years, the antibody pattern changed to chronic Q fever with the presence of high phase 1 IgG and IgA titers (Table 2).
Table 1.
Q fever diagnosis of patients by immunofluorescence serology
| No. of sera and serology tests per patient | No. of patients tested | No. of patients who seroconverted | No. of patients who seroconverted to only P2 Coxiella burnetii antigens | No. of patients who seroconverted to P1 and P2 C. burnetii antigens |
|---|---|---|---|---|
| 1 | 40 | N/A | N/A | N/A |
| 2 | 55 | 26 | 7 | 19 |
| 3 | 41 | 20 | 6 | 14 |
| 4 | 11 | 3 | 0 | 3 |
| 5 | 7 | 2 | 0 | 2 |
| 6 | 2 | 0 | 0 | 0 |
| 8 | 2 | 0 | 0 | 0 |
| 9 | 1 | 0 | 0 | 0 |
| 10 | 1 | 0 | 0 | 0 |
| Total | 160 | 51 | 13 | 38 |
N/A = not applicable; P1 = phase 1; P2 = phase 2.
Table 2.
Q fever immunofluorescence serological profiles of acute and chronic Q fever patients
| Infection type and patient number | Day of sample* | Antibody to phase 2 | Antibody to phase 1 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Coxiella burnetii antigen | C. burnetii antigen | ||||||||
| IgM | IgG | IgA | Total | IgM | IgG | IgA | Total | ||
| Acute Q fever | |||||||||
| 174 (adult) | 0 | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg |
| 14 | ≥ 3,200 | 800 | Neg | ≥ 3,200 | Neg | Neg | Neg | Neg | |
| 44 | 400 | ≥ 3,200 | 400 | ≥ 3,200 | 200 | 200 | 200 | 200 | |
| 176 (adult) | 0 | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg |
| 1 | 50 | 25 | Neg | 50 | Neg | Neg | Neg | Neg | |
| 15 | ≥ 3,200 | ≥ 3,200 | Neg | ≥ 3,200 | 100 | Neg | Neg | 100 | |
| 267 (adult) | 0 | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg |
| 4 | ≥ 3,200 | 100 | 50 | ≥ 3,200 | Neg | Neg | Neg | Neg | |
| 11 | ≥ 3,200 | ≥ 3,200 | 50 | ≥ 3,200 | 200 | Neg | Neg | 200 | |
| Chronic Q fever | |||||||||
| 271 (child: chronic osteomyelitis) | 0 | Neg | ≥ 3,200 | Neg | ≥ 3,200 | Neg | ≥ 3,200 | 200 | ≥ 3,200 |
| 198 | Neg | ≥ 3,200 | Neg | ≥ 3,200 | Neg | ≥ 3,200 | 200 | ≥ 3,200 | |
| 438 | Neg | ≥ 3,200 | 50 | ≥ 3,200 | Neg | ≥ 3,200 | 400 | ≥ 3,200 | |
| 243 (adult: chronic endocarditis) | 0 | Neg | ≥ 3,200 | ≥ 3,200 | ≥ 3,200 | 50 | ≥ 3,200 | ≥ 3,200 | ≥ 3,200 |
| 446 | Neg | ≥ 3,200 | ≥ 3,200 | ≥ 3,200 | Neg | ≥ 3,200 | ≥ 3,200 | ≥ 3,200 | |
| 1,026 | Neg | 800 | 100 | 800 | Neg | 1,600 | 800 | 1,600 | |
| 199† (adult: acute to chronic endocarditis) | 0 | ≥ 3,200 | Neg | Neg | ≥ 3,200 | Neg | Neg | Neg | Neg |
| 10 | ≥ 3,200 | ≥ 3,200 | 200 | ≥ 3,200 | Neg | Neg | Neg | Neg | |
| 1,825 (5 years) | Neg | 1,600 | Neg | 1,600 | Neg | ≥ 3,200 | ≥ 3,200 | ≥ 3,200 | |
Neg = negative.
The day 0 sample would usually be within 1 week of onset of illness.
Patient 199 was first diagnosed as acute Q fever, and 5 years later, represented with chronic Q fever.
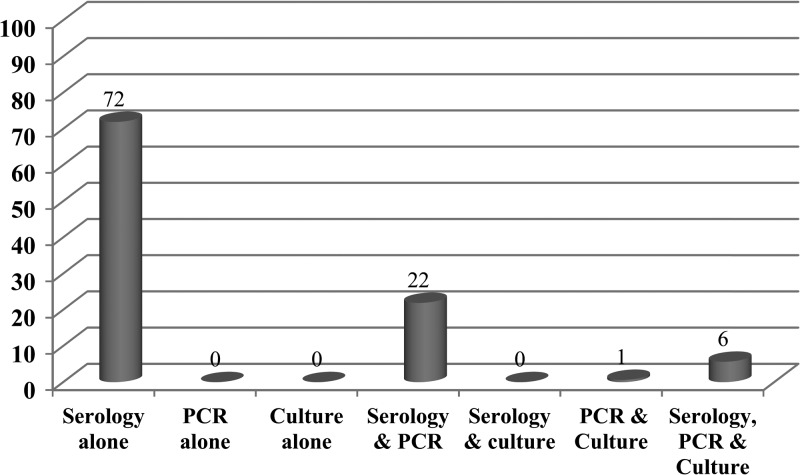
In 14 of the 160 patients, C. burnetii was isolated from the patient serum by VERO cell monolayer inoculation. This cohort consisted of 13 acute cases and one chronic case. Of these 14 cases for whom PCR was performed, only six were positive (43% sensitivity), showing that PCR was a valuable diagnostic technique only if positive. Given that two culture-positive cases were qPCR (false) negative, it is apparent that the qPCR assay lacked sensitivity compared with amplification by culture. However, qPCR is infinitely faster and when positive, of much greater use to the doctor. Serology alone (72%) was the most commonly used laboratory assay, followed by serology and PCR (22%). In 6% of patients' serology, PCR and culture of C. burnetii constituted the diagnosis (Figure 4).
Figure 4.
Distribution (%) of different laboratory technique(s) used for the diagnosis of Q fever patients (n = 160).
Of the 135 patients in whom the doctor reported the use of antibiotics, 130 (96%) used doxycycline. Of these patients, 126 (97%) were reported as cured. The remainder were probably still fatigued due to the post-Q fever fatigue syndrome.
DISCUSSION
Australia could be considered the “home” of Q fever: the infection was first recognized as a new clinical entity among abattoir workers slaughtering pregnant cattle in Brisbane, Australia,1 recognized as a probable Rickettsia,11 its epidemiology was defined12 and a clinical analysis was undertaken.13 In the United States, the bacterium was detected in ticks2 and shown to be a human pathogen.14 In the intervening years, Q fever has been shown to be widespread in the world, an important zoonosis involving many vertebrate animal species and tick species, with recent refinements in diagnosis and treatment.15,16 Unfortunately Australia is still the only country using a human Q fever vaccine.17 A large French study18 has provided benchmark data. But could the disease in Australia be different? There have been previous epidemiological studies of Q fever in Queensland,5,6,12,13,19–22 Victoria,7,8 and comparisons,23,24 but there are limited data available on Q fever in NSW.25–28 A seroprevalence study in the Hunter New England (northeast) region of NSW showed a seroprevalence range from 0.5% in the city (Newcastle) to a maximum of 22% in a rural area.29 A 10-year summary (2001–2010) of the NSW official Q fever notification data30 overlapped with the current study (2005–2013). In both studies, similar conclusions were noted, including the predominance of cases in rural NSW, in middle-aged males. The use of the Q fever vaccine as part of a government sponsored industry immunization program reduced the incident of Q fever over a decade from 4.5 to 2.8 cases per 100,000 persons per year.30 The paucity of Q fever cases in children has been already reported in Australia,31,32 but when it does occur, chronic osteomyelitis is the main clinical presentation.33,34
One important new finding from the current study is the role of macropods (especially kangaroos) in the epidemiology of Q fever in Australia. Several patients appear to have been infected via indirect contact with kangaroos, either via their feces or via their ticks. This link has been postulated previously.35 Two species of kangaroos in western Queensland were shown to be seropositive for C. burnetii, and isolates of C. burnetii were made from their ticks, Amblyomma triguttatum, the ornate kangaroo's tick.36 A patient in western Australia developed Q fever pericarditis after being bitten by kangaroo ticks.37 Macropod seropositivity to C. burnetii in Queensland and western Australia has been reported.38 Several Australian tick species have been shown to contain C. burnetii DNA39 including A. triguttatum (from macropods) and Haemaphysalis humerosa (from bandicoots, which are known to be an important reservoir for C. burnetii). Ixodes holocyclus, the paralysis tick, which is the main human-biting tick in Australia and Bothriocroton auruginans, the wombat tick, also harbor C. burnetii. It is now beyond reasonable doubt that kangaroos and other macropods and their ticks carry C. burnetii. Kangaroo feces may pose a risk of Q fever especially if aerosolized by wind or lawn mowing. In the current study, 7% of patients remembered macropods contact.
In patients with acute Q fever, phase 2 IgG appeared marginally sooner than phase 1 IgM. In patients with chronic Q fever, phase 1 IgA titers were generally higher than phase 2 IgA titers. The unusual acute-to-chronic case (no. 199) showed the stark serological differences between the two forms of the illness. The value the PCR in early Q fever diagnosis has already been reported in Australia,40 but the observation that culture was more sensitive than qPCR was an unexpected finding.
The myth that Australian Q fever patients do not have respiratory symptoms has also been overturned (Figure 2). Early studies on Q fever in Australia had shown that 69% of patients had a cough.41 The current study has shown that hepatitis is more common than respiratory symptoms although many patients had both.
In summary, this study of 160 cases from a 379 case cohort of Q fever in NSW has demonstrated that much about this infectious disease in Australia is similar to elsewhere in the world, with a background endemicity associated with native wildlife (macropods in the case of Australia), and the main burden being due to livestock industries particularly cattle and sheep. Increased uptake of the Q fever vaccine by young adults (≥ 18 years), in rural Australia, should be encouraged.28
ACKNOWLEDGMENTS
We thank John Stenos, Gemma Vincent, Chelsea Nguyen, and other colleagues at the Australian Rickettsial Reference Laboratory, Geelong, Australia, for excellent scientific assistance. We extend sincere thanks also to all participating doctors and their patients who provided clinical information through the questionnaire.
Footnotes
Financial support: We are grateful to Rod Givney, John Ferguson, Anne Crotty, and Mark Formby of NSW Health Pathology, Newcastle, Australia, for valuable financial support for this work.
Authors' addresses: Stephen R. Graves, Australian Rickettsial Reference Laboratory, Newcastle Branch, New South Wales, Australia, E-mail: rickettsia@gmail.com. Aminul Islam, Department of Microbiology, Hunter Area Pathology Service, John Hunter Hospital, New South Wales, Australia, E-mail: aminul.islam@hnehealth.nsw.gov.au.
References
- 1.Derrick EH. “Q” fever, a new fever entity: clinical features, diagnosis and laboratory investigation. Med J Aust. 1937;21:281–299. doi: 10.1093/clinids/5.4.790. [DOI] [PubMed] [Google Scholar]
- 2.Davis GE, Cox HR, Parker RR, Dyer RE. A filter-passing infectious agent isolated from ticks. 1. Isolation from Dermacentor andersoni, reactions in animals and filtration experiments. Public Health Rep. 1938;53:2259–2282. [Google Scholar]
- 3.Hilbink F, Penrose M, Kovacova E, Kazar J. Q fever is absent from New Zealand. Int J Epidemiol. 1993;22:945–949. doi: 10.1093/ije/22.5.945. [DOI] [PubMed] [Google Scholar]
- 4.Musso D, Broult J, Parola P, Raoult D, Fournier PE. Absence of antibodies to Rickettsia spp, Bartonella spp., Ehrlichia spp. and Coxiella burnetii in Tahiti, French Polynesia. BMC Infect Dis. 2014;14:255–259. doi: 10.1186/1471-2334-14-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Powell O. “Q” fever: clinical features in 72 cases. Australas Ann Med. 1960;9:214–223. doi: 10.1111/imj.1960.9.3.214. [DOI] [PubMed] [Google Scholar]
- 6.Derrick EH. The course of infection with Coxiella burnetii. Med J Aust. 1973;1:1051–1057. [PubMed] [Google Scholar]
- 7.Spelman DW. Q fever: a study of 111 consecutive cases. Med J Aust. 1982;1:547–553. doi: 10.5694/j.1326-5377.1982.tb124169.x. [DOI] [PubMed] [Google Scholar]
- 8.Buckley B. Q fever epidemic in Victorian general practice. Med J Aust. 1980;1:593–595. doi: 10.5694/j.1326-5377.1980.tb135159.x. [DOI] [PubMed] [Google Scholar]
- 9.Graves S, Stenos J, Unsworth N, Nguyen C. Laboratory diagnosis of rickettsial infection. Aust J Med Sci. 2006;27:39–44. [Google Scholar]
- 10.Stenos J, Graves S, Lockhart M. Chapter 14: Coxiella burnetii. In: Carter IWJ, Schuller M, James GS, Sloots TP, Halliday CL, editors. PCR for Clinical Microbiology. Berlin, Germany: Springer Science + Business Media BV; 2010. pp. 145–148. [Google Scholar]
- 11.Burnet FM, Feeman M. Experimental studies on the virus of “Q” fever. Med J Aust. 1937;21:299–305. [Google Scholar]
- 12.Derrick EH. The epidemiology of Q fever. J Hyg (Lond) 1944;43:357–361. doi: 10.1017/s0022172400013085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Derrick EH. The epidemiology of “Q” fever: a review. Med J Aust. 1953;1:245–253. [PubMed] [Google Scholar]
- 14.Dyer RE. A filter-passing agent isolated form ticks. IV. Human infection. Public Health Rep. 1938;53:2277–2282. [Google Scholar]
- 15.Maurin M, Raoult D. Q fever. Clin Microbiol Rev. 1999;12:518–553. doi: 10.1128/cmr.12.4.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson A, Bijlmer H, Founier PE, Graves S, Hartzell J, Kersh GJ, Limonard G, Marrie TJ, Massung RF, McQuiston JH, Nicholson WL, Paddock CD, Sexton DJ. Diagnosis and management of Q fever—United States, 2013: recommendations from the CDC and the Q fever working group. MMWR Recomm Rep. 2013;62:1–30. [PubMed] [Google Scholar]
- 17.Marmion B. Q fever: the long journey to control by vaccination. Med J Aust. 2007;186:164–166. doi: 10.5694/j.1326-5377.2007.tb00853.x. [DOI] [PubMed] [Google Scholar]
- 18.Raoult D, Tissot-Dupont H, Foucault C, Gouvernet J, Fournier PE, Bernit E, Stein A, Nesri M, Harle JR, Weiller PJ. Q fever 1985–1998. Clinical and epidemiologic features of 1,383 infections. Medicine (Baltimore) 2000;79:109–123. doi: 10.1097/00005792-200003000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Johnson DW. Epidemiology of Q fever in Queensland: a seven-year survey. Med J Aust. 1966;1:121–125. doi: 10.5694/j.1326-5377.1966.tb19554.x. [DOI] [PubMed] [Google Scholar]
- 20.Boland PJ, Parker NR. Q fever in south west Queensland. Med J Aust. 1999;171:446. doi: 10.5694/j.1326-5377.1999.tb123737.x. [DOI] [PubMed] [Google Scholar]
- 21.Harris P, Eales KM, Squires R, Govan B, Norton R. Acute Q fever in northern Queensland: variation in incidence related to rainfall and geographical location. Epidemiol Infect. 2013;141:1034–1038. doi: 10.1017/S0950268812001495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paymard M, Nicotra L, Dettrick A, Bell B, Chaudhuri A, Cody D, Tesar P, Hamilton-Craig C. Chronic Q fever prosthetic valve endocarditis—an important cause of prosthetic valve dysfunction in Australia. Med J Aust. 2015;202:212–213. doi: 10.5694/mja14.00864. [DOI] [PubMed] [Google Scholar]
- 23.Garner MG, Longbottom HM, Cannon RM, Plant AJ. A review of Q fever in Australia 1991–1994. Aust N Z J Public Health. 1997;21:722–730. doi: 10.1111/j.1467-842x.1997.tb01787.x. [DOI] [PubMed] [Google Scholar]
- 24.Australian Government Department of Health and Ageing Vaccine preventable diseases in Australia, 2005 to 2007. Q fever. Communicable Diseases Intelligence. 2010;34:S84–S93. [PubMed] [Google Scholar]
- 25.Duggan JM, Hunter WF. Q fever: a report of three cases. Med J Aust. 1956;43:645–647. [PubMed] [Google Scholar]
- 26.Henson J, Hansman D. Q fever among abattoir workers in New South Wales. Med J Aust. 1963;50:343–346. doi: 10.5694/j.1326-5377.1963.tb23065.x. [DOI] [PubMed] [Google Scholar]
- 27.Massey P, Taylor K. Q fever cluster in a shearing team. N S W Public Health Bull. 2004;15:11–12. [Google Scholar]
- 28.Karki S, Gidding H, Newall T, McIntyre P, Liu B. Risk factors and burden of acute Q fever in older adults in New South Wales: a prospective cohort study. Med J Aust. 2015;203:438–448. doi: 10.5694/mja15.00391. [DOI] [PubMed] [Google Scholar]
- 29.Islam A, Ferguson J, Givney R, Graves S. Seroprevalence to Coxiella burnetii among residents of the Hunter New England region of New South Wales, Australia. Am J Trop Med Hyg. 2011;84:318–320. doi: 10.4269/ajtmh.2011.10-0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lowbridge CP, Tobin S, Seale H, Ferson MJ. EpiReview: notification of Q fever in NSW, 2001–2010. N S W Public Health Bull. 2012;23:31–35. doi: 10.1071/NB11037. [DOI] [PubMed] [Google Scholar]
- 31.Massey P, Irwin M, Durrheim D. Enhanced Q fever risk exposure surveillance may permit better informed vaccination policy. Commun Dis Intell Q Rep. 2009;33:41–45. [PubMed] [Google Scholar]
- 32.Parker N, Robson J, Bell M. A serosurvey of Coxiella burnetii infection in children and young adults in south west Queensland. Aust N Z J Public Health. 2010;34:79–82. doi: 10.1111/j.1753-6405.2010.00478.x. [DOI] [PubMed] [Google Scholar]
- 33.Nourse C, Allworth A, Jones A, Horvath R, McCormack J, Bartlett J, Hayes D, Robson JM. Three cases of Q fever osteomyelitis in children and a review of the literature. Clin Infect Dis. 2004;39:e61–e66. doi: 10.1086/424014. [DOI] [PubMed] [Google Scholar]
- 34.Britton PN, Macartney K, Arbuckle S, Little D, Kesson A. A rare case of Q fever osteomyelitis in a child form regional Australia. J Pediatric Infect Dis Soc. 2015;4:e28–e31. doi: 10.1093/jpids/piu095. [DOI] [PubMed] [Google Scholar]
- 35.Derrick EH, Pope JH, Smith DJW. Outbreaks of Q fever in Queensland associated with sheep. Med J Aust. 1959;46:585–588. [PubMed] [Google Scholar]
- 36.Pope JH, Scott W, Dwyer R. Coxiella burnetii in kangaroos and kangaroo ticks in western Queensland. Aust J Exp Biol. 1960;38:17–27. doi: 10.1038/icb.1960.3. [DOI] [PubMed] [Google Scholar]
- 37.Beaman M, Hung J. Pericarditis associated with tick-borne Q fever. Aust N Z J Med. 1989;19:254–256. doi: 10.1111/j.1445-5994.1989.tb00258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cooper A, Barnes T, Potter A, Ketheesan N, Govan B. Determination of Coxiella burnetii seroprevalence in macropods in Australia. Vet Microbiol. 2012;155:317–323. doi: 10.1016/j.vetmic.2011.08.023. [DOI] [PubMed] [Google Scholar]
- 39.Cooper A, Stephens J, Ketheesan N, Govan B. Detection of Coxiella burnetii DNA in wildlife and ticks in northern Queensland, Australia. Vector Borne Zoonotic Dis. 2013;13:12–16. doi: 10.1089/vbz.2011.0853. [DOI] [PubMed] [Google Scholar]
- 40.Turra M, Chang G, Whybrow D, Higgins G, Qiao M. Diagnosis of acute Q fever by PCR on sera during a recent outbreak in rural south Australia. Ann N Y Acad Sci. 2006;1078:566–569. doi: 10.1196/annals.1374.112. [DOI] [PubMed] [Google Scholar]
- 41.Gilroy N, Formica N, Beers M, Egan A, Conaty S, Marmion B. Abattoir-associated Q fever: a Q fever outbreak during a Q fever vaccination program. Aust N Z J Public Health. 2001;25:362–367. doi: 10.1111/j.1467-842x.2001.tb00595.x. [DOI] [PubMed] [Google Scholar]