Figure 2.
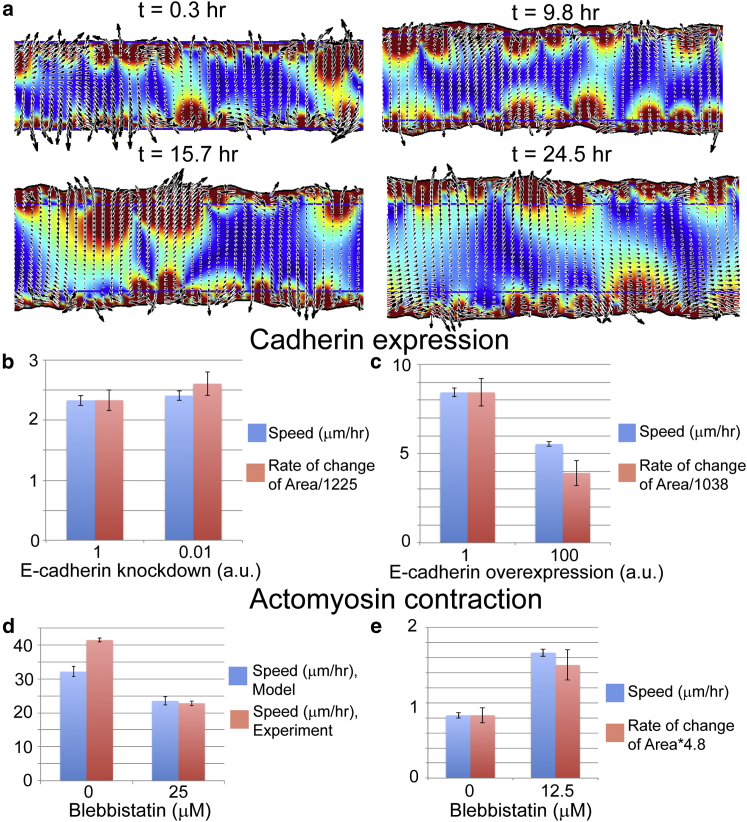
(a) We validated our model by comparing simulations with previous wound healing experiments that perturbed cell-cell adhesion and actomyosin contraction in cancer-relevant cell lines. The wound healing simulations compute the velocity field in a strip of epithelial cells. We track the spreading of a strip of cells and measure the average rate of the border advance. Overexpression of N-cadherin leads to decreased levels of E-cadherin and was shown to produce a small increase in the time rate of change of the area of a wounded layer of MCF10A cells (b, red bars), whereas knockdown of N-cadherin reduced E-cadherin levels and slowed the progression of the area (c, red bars) (data for the time rate of change of the area dA/dt taken from (33) and scaled to compare with simulation velocities). Our model also shows that decreasing the cell-cell adhesion (the cell-cell viscosity η) produces a modest increase in the advance of the border (b, blue bars), whereas increasing cell-cell adhesion slows border advance in our simulations (c, blue bars). (d and e) Blebbistatin treatment differentially affects different cell types. MCF10A cells on rigid substrate migrate rapidly and are slowed down when blebbistatin is used to inhibit actomyosin contraction (34) (d, red bars). Consistent with this finding, our model produces a decrease in speed when we decrease the dipole stress for parameters that give fast migration (d, blue bars). On the other hand, border speed in slower cells, such as hepatic cells, is observed to increase upon blebbistatin treatment (33), which agrees with our model when we use parameters that reduce the overall border migration (e, red bars are data from (33); blue bars are simulation results). See text and Table 1 for parameters and details. To see this figure in color, go online.