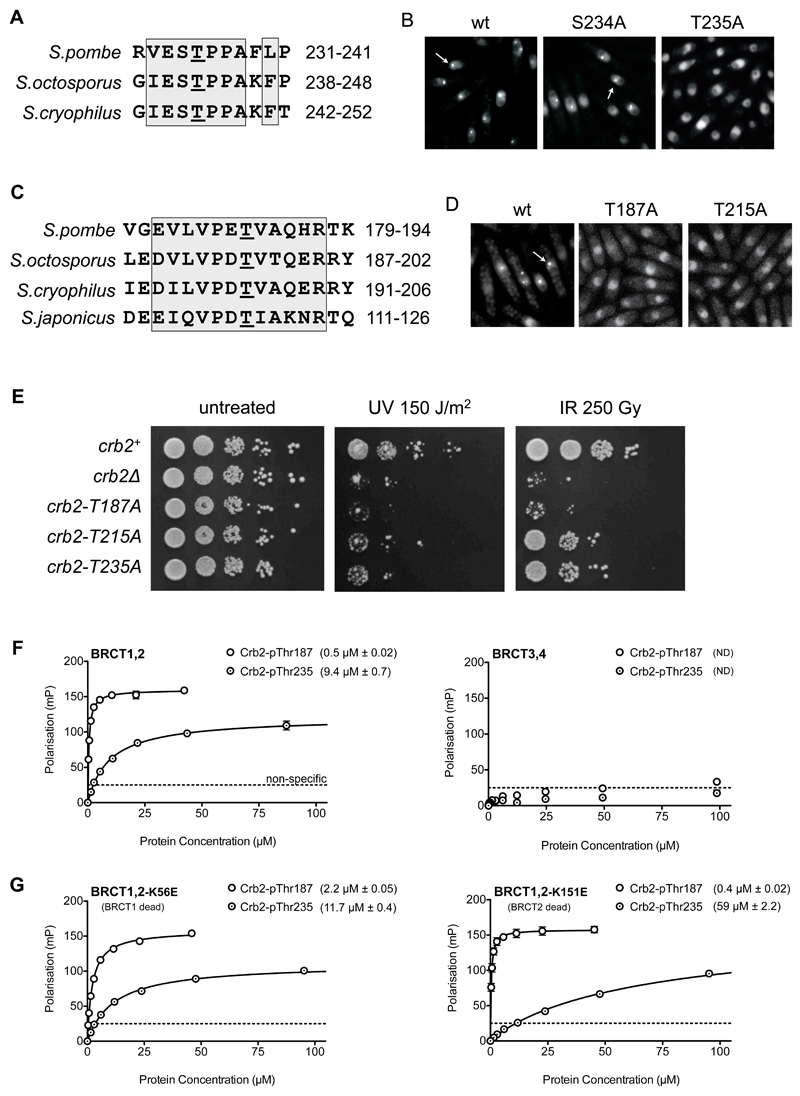
Figure 3. Identification of Crb2 phosphorylation sites mediating interaction with Rad4.
A) Thr235 is a consensus CDK phosphorylation site conserved across Schizosaccharomyces species.
B) Compared to wild-type (left) or an S234A mutation (middle), T235A mutation abolished recruitment of a YFP-tagged Crb2(67-358)-LZ reporter to DNA double-strand breaks (see METHODS). Punctate fluorescent foci are indicated by arrows. In all focus formation figures, cells were exposed to 80 Gray of ionising radiation.
C) Truncation analysis (SUPPLEMENTARY FIGURE 1) identifies a Crb2 segment, from amino acids 180 to 200, as essential for DNA damage focus formation by the fluorescent Crb2 reporter, with Thr187 being the only conserved phosphorylatable residue in the vicinity.
D) Mutation of Thr187 or Thr215 also abolished recruitment of the fluorescent Crb2 reporter to DNA damage foci.
E) Alanine mutation of Thr187, Thr215 or Thr235 all impaired survival of exposure to UV or ionizing radiation, compared to unmutated Crb2 (see METHODS). T215A and T235A displayed some residual resistance whereas T187A phenocopied the Crb2 null strain.
F) A Crb2-derived peptide incorporating pThr187 bound with sub-micromolar affinity to Rad4-BRCT1,2, but not BRCT3,4. A pThr235 peptide bound with modest micromolar affinity to Rad4-BRCT1,2, but not BRCT3,4. Data points are averages of three measurements; error bars indicate one standard deviation.
G) Charge-reversal mutation of the phosphate-binding site in either BRCT1 (left) or BRCT2 (right) did not significantly affect the tight binding of the Crb2-pThr187 peptide, suggesting that it is able to bind to either site. By contrast, the modest affinity of the Crb2-pThr235 peptide was decreased 5-fold by a BRCT2 mutation, identifying this as its preferred binding site. Data points are averages of three measurements; error bars indicate one standard deviation.