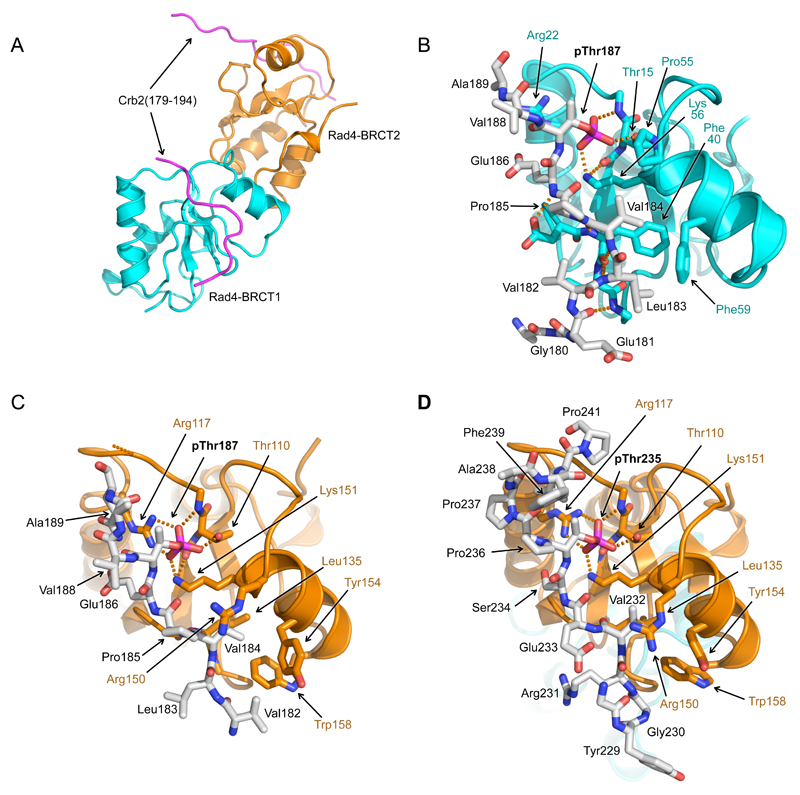
Figure 4. Structure of Rad4 – Crb2 phosphopeptide complexes.
A) Overall structure of Rad4-BRCT1,2 bound to two copies of a Crb2-derived peptide incorporating pThr187. BRCT1 is coloured cyan, BRCT2 orange, and Crb2 peptides in magenta.
B) Detail of interaction of Crb2-pThr187 peptide with Rad4-BRCT1. The phosphate group of pThr187 interacts with the side-chain of Lys56 and the main and side chains of Thr15. Upstream of pThr187, Val184 binds in a hydrophobic pocket on the BRCT domain. This orientation of peptide binding to a BRCT domain has not previously been observed.
C) As B) but for Rad4-BRCT2. As well as Lys151 and Thr110, the phosphate of Crb2-pThr187 also interacts with Arg117 of Rad4. The peptide binds in the same orientation as to BRCT1, with Val184 again interacting with a hydrophobic pocket.
D) As C) but for a Crb2-derived peptide incorporating pThr235. Consistent with the measured affinities the pThr235 peptide was only found bound to BRCT2 in the crystal structure. The peptide binds in the same orientation as the pThr187 peptide, with the phosphate group and the upstream valine residue (Val232) making the same interactions as in the pThr187 peptide complex.