Figure 4.
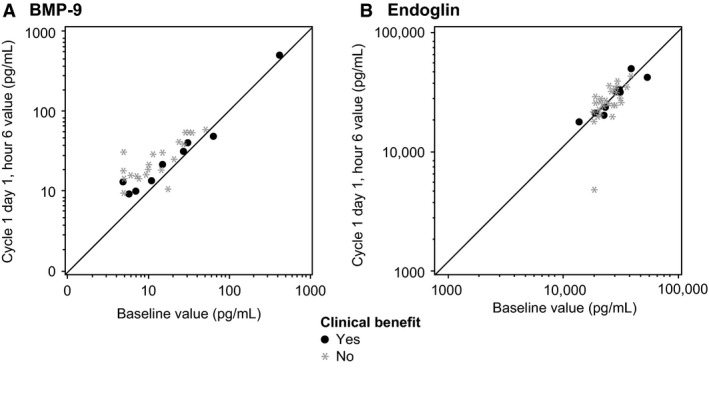
Individual circulating levels of (A) bone morphogenetic proteins (BMP)‐9 and (B) soluble endoglin detected in patients at baseline and 6 h postinfusion of PF‐03446962 (day 1, cycle 1), according to treatment response. Clinical benefit includes complete or partial responses and stable disease lasting for ≥84 days.
