FIGURE 10:
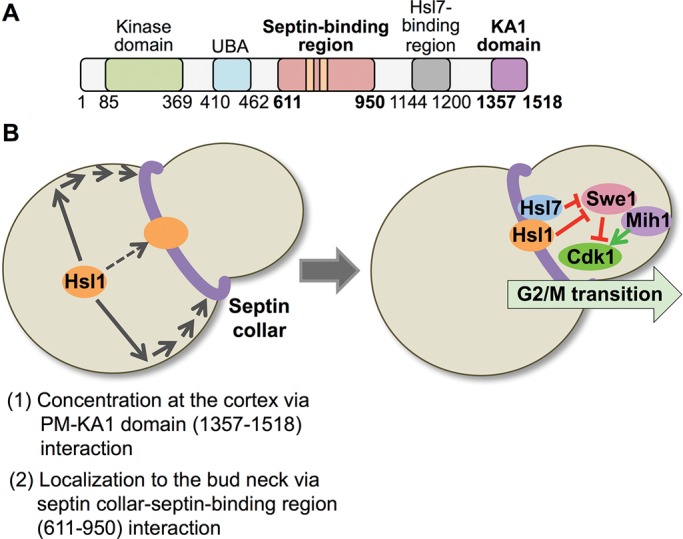
Two-step mechanism for localization of Hsl1 to the septin collar at the bud neck. (A) Diagram of Hsl1 with the locations of the septin-binding region (pink) pinpointed in this study and the KA1 domain (purple) highlighted. As described in the text, the 611–950 segment contains two distinct and separable septin-association elements, as well as the KEN and D-box APC recognition motifs (orange bars). Residues 611–950 are both necessary and sufficient for binding of Hsl1 to the septin filaments in the collar at the bud neck; however, for optimal and exclusive recruitment of Hsl1 to this location, interaction of the KA1 domain with the PM is required. (B) Two-stage model for efficient recruitment of Hsl1 to the septin collar. Left, at the bud neck, septin filaments are tightly associated with the PM via their binding to PtdIns4,5P2, a lipid enriched at the bud neck. The KA1 domain concentrates Hsl1 at the PM by binding to PtdSer, another lipid that is also enriched at the bud neck. Thus the most likely scenario to account for the observed synergy between the KA1 domain and the 611–950 septin-binding segment is that KA1-mediated concentration of Hsl1 at the PM permits more efficient encounter of its 611–950 septin-binding domain with the septin filaments. Right, subsequent Hsl1-dependent recruitment of Hsl7 to the bud neck promotes degradation of Swe1, a negative regulator of Clb-bound Cdk1/Cdc28, which, in conjunction with the action of the phosphatase Mih1, fully releases Cdk1 from inhibition, allowing for timely initiation of and passage through the G2/M transition of the cell cycle.
