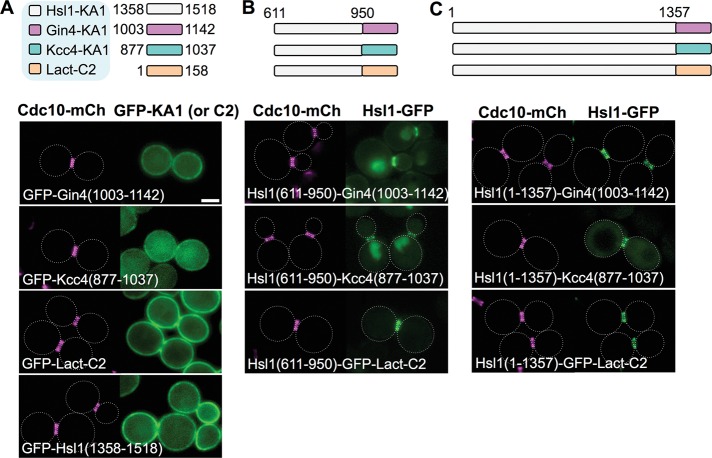
FIGURE 7:
High-affinity PM binding is the sole role of the KA1 domain for localization of Hsl1 to the septin collar. (A) Top, diagram of the KA1 domains of the indicated protein kinases (white, Hsl1; pink, Gin4; and, blue, Kcc4) and the C2 domain of bovine (Bos taurus) lactadherin (orange), each fused to the C-terminus of eGFP, that were examined. Bottom, plasmids producing the indicated constructs (pGF-IVL181, pGF-IVL184, pGF-IVL187, and pGF-IVL708), expressed and visualized as in Figure 1, A and B. (B) Top, diagram of the fusions of the 611–950 segment of Hsl1 to the indicated heterologous membrane-targeting domains (and to eGFP) that were examined. Bottom, plasmids producing the indicated constructs (pGF-IVL639, pGF-IVL641 and pGF-IVL688), expressed and visualized as in Figure 1, A and B. (C) Top, diagram of the constructs in which the endogenous KA1 in Hsl1 was substituted with the indicated heterologous membrane-targeting domains (fused to eGFP). Bottom, plasmids producing the indicated constructs (pGF-IVL638, pGF-IVL640 and pGF-IVL687), expressed and visualized as in Figure 1, A and B. Dotted white line, cell periphery; scale bar, 2 μm. The dotted white line is omitted for constructs that exhibited significant PM fluorescence.