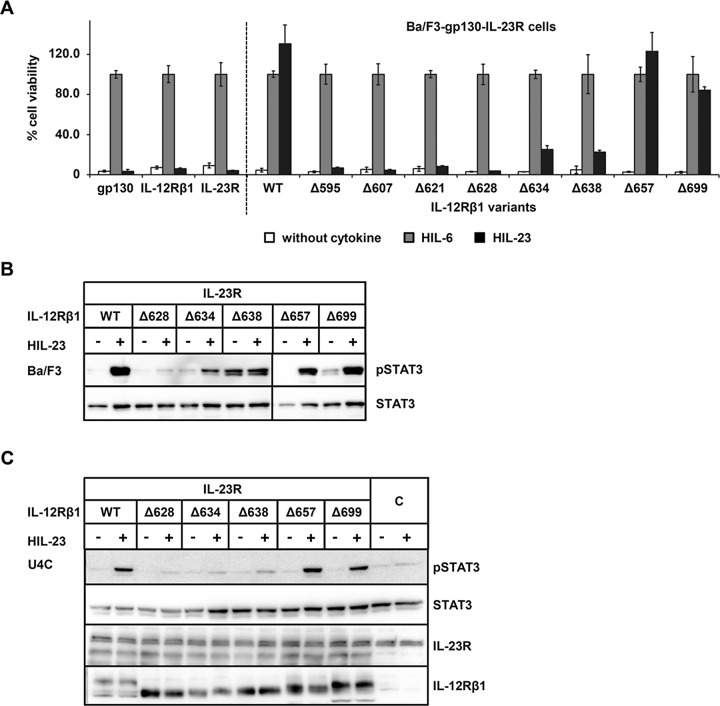
FIGURE 3:
The cytoplasmic domain of mouse IL‑12Rβ1 is important for STAT3 activation. (A) Proliferation of stably cotransduced Ba/F3-gp130 cells with cDNAs coding for murine IL‑23R and murine IL‑12Rβ1 (WT) or appropriate deletion variants. Equal numbers of cells were cultured for 3 d in the presence of 0.2% HIL‑6–conditioned cell culture supernatant or 0.2% HIL‑23 or without cytokine. Parental Ba/F3-gp130 cells were used as control. Proliferation was measured using the colorimetric CellTiter-Blue Cell Viability Assay. HIL‑6–dependent proliferation was set to 100%. One representative experiment is shown. Error bars represent SD for technical replicates. (B) Stably transduced Ba/F3 cells were washed three times, starved, and stimulated with 0.2% HIL‑23 for 30 min. Cellular lysates were prepared, and equal amounts of total protein (50 μg/lane) were loaded on SDS gels, followed by immunoblotting using specific antibodies for phospho-STAT3 and STAT3. Western blot data show one representative experiment out of three. (C) U4C cells were transiently transfected with cDNAs for murine IL‑23R and IL‑12Rβ1 variants. At 30 h after transfection, cells were washed with PBS and starved overnight in serum-free medium. Cells were then stimulated with HIL‑23 for 30 min. Cellular lysates were prepared, and equal amounts of total protein (50 μg/lane) were loaded on SDS gels, followed by immunoblotting using specific antibodies for phospho-STAT3, STAT3, mIL-23R, and mIL-12Rβ1. Nontransfected U4C cells served as negative control (C). Western blot data show one representative experiment out of three.