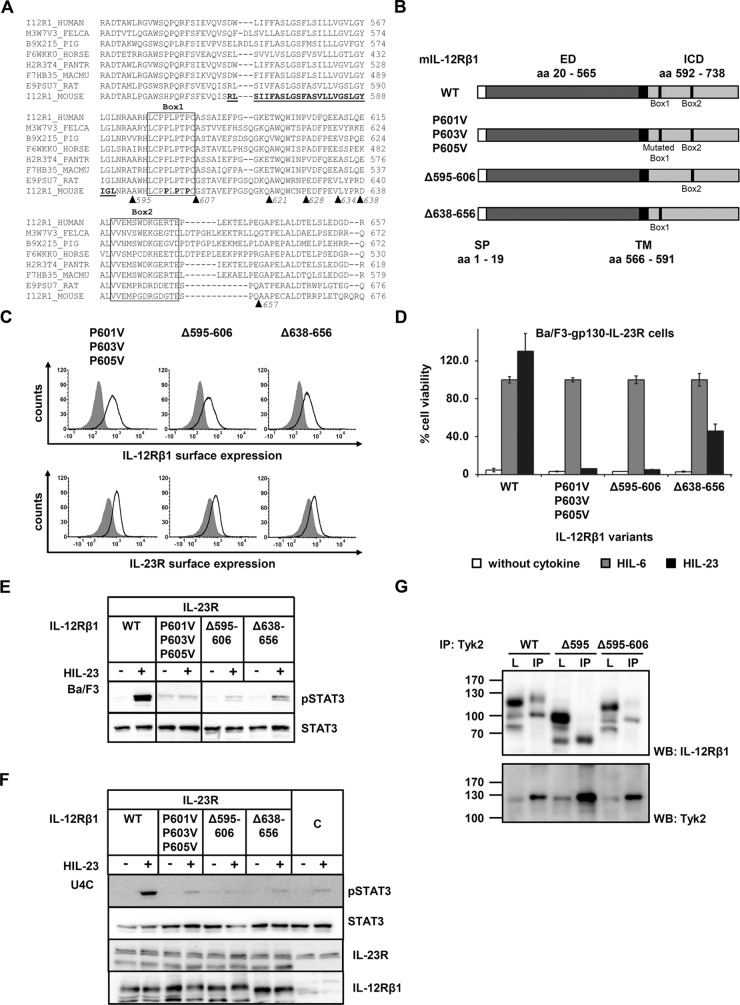
FIGURE 4:
Association of Tyk2 with IL-12Rβ1 via the Box1 motif is mandatory for IL-23 signal transduction. (A) Aligned amino acid sequences of IL‑12Rβ1. The transmembrane domain of the mouse IL‑12Rβ1 is highlighted in bold. Box1 and Box2 motifs are as described in Chua et al. (1995). Deletion variants are depicted by triangles. (B) IL‑12Rβ1 variants with mutated or deleted Box motifs were generated by PCR. The extracellular (ED) and the transmembrane domain (TM) of IL-12Rβ1 are unaffected. (C) Representative histograms of IL‑12Rβ1 (top) and IL‑23R (bottom) surface expression of stably transduced Ba/F3-gp130 cell lines. Gray-shaded areas indicate Ba/F3-gp130 cells (negative control), and dark solid lines are the respective Ba/F3 cell lines as indicated. (D) Proliferation of stably cotransduced Ba/F3-gp130 cells with cDNAs coding for murine IL‑23R and murine IL‑12Rβ1 Box1 or Box2 deletion variants or mutant IL‑12Rβ1. Equal numbers of cells were cultured for 3 d in the presence of 0.2% HIL‑6 or 0.2% HIL‑23 or without cytokine. Ba/F3-gp130 cells expressing murine IL‑23R and IL‑12Rβ1 were used as control. Proliferation was measured using the colorimetric CellTiter-Blue Cell Viability Assay, and HIL‑6–dependent proliferation was set to 100%. Error bars represent SD for technical replicates. (E) Stably transduced Ba/F3 cells were washed three times, starved, and stimulated with 0.2% HIL‑23 for 30 min. Cellular lysates were prepared, and equal amounts of total protein (50 μg/lane) were loaded on SDS gels, followed by immunoblotting using specific antibodies for phospho-STAT3 and STAT3. Western blot data show one representative experiment out of three. (F) U4C cells were transiently transfected with cDNAs for murine IL‑23R and IL‑12Rβ1 deletion and mutant variants. Cotransfected U4C cells expressing wild-type receptors were used as control. At 30 h after transfection, cells were washed with PBS and starved overnight in serum-free medium. Cells were then stimulated with HIL‑23 for 30 min. Cellular lysates were prepared, and 50 μg of total protein per lane was loaded on SDS gels, followed by immunoblotting using specific antibodies for phospho-STAT3, STAT3, mIL-23R, and mIL-12Rβ1. Nontransfected U4C cells served as negative control (C). Western blot data show one representative experiment. (G) The Box1 motif of IL‑12Rβ1 is important for the association of Tyk2. COS-7 cells were cotransfected with cDNAs coding for murine Tyk2 and full-length IL‑12Rβ1, a deletion variant lacking Box1 motif, or an IL‑12Rβ1 variant without the cytoplasmic domain. Tyk2 was immunoprecipitated, and Western blot analysis was performed to detect the appropriate IL-12Rβ1 variant and Tyk2. Three independent experiments were performed, and one representative experiment is shown. IP, Tyk2 coimmunoprecipitation; L, lysate.