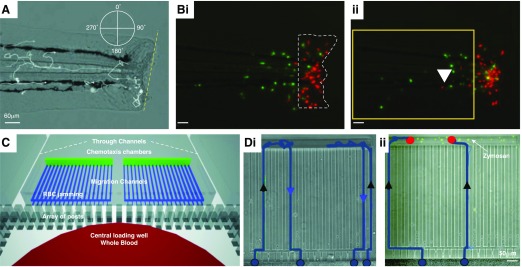
Figure 3. Modeling of inflammation resolution by neutrophil reverse migration.
(A) A transgenic zebrafish model of sterile inflammation. Neutrophils are labeled by GFP expression, driven by the myeloperoxidase promoter, allowing in vivo tracking of neutrophil migration relative to the wound (dashed yellow line) during recruitment and resolution phases. (B, i) Reverse-migrating neutrophils can be more easily visualized by spatially restricted photoconversion (from green to red) of wound-associated neutrophils (dashed white outline) that express the fluorescent protein Kaede. (ii) Reverse migration of neutrophils can then be monitored by identifying those cells that return to the tissue (white arrowhead). [A and B reproduced from Robertson, A. L., Holmes, G. R., Bojarczuk, A. N., Burgon, J., Loynes, C. A., Chimen, M., Sawtell, A. K., Hamza, B., Willson, J., Walmsley, S. R., Anderson, S. R. (2014) A zebrafish compound screen reveals modulation of neutrophil reverse migration as an anti-inflammatory mechanism. Sci Transl Med 6, 225ra29–225ra29. Reprinted with permission from AAAS.] (C) A microfluidic device to measure neutrophil recruitment and reverse migration from a drop of whole blood. (D, i) Neutrophils (blue tracks) are recruited (black arrowheads) from the whole blood to the chemotaxis chambers and then reverse migrate (blue arrowheads) down the chemokine gradient and back into the whole-blood reservoir. (ii) In the presence of microbial particles (zymosan; green), neutrophils are retained (red dots) at the chemokine reservoir and show reduced reverse migration. [C and D reproduced from Hamza, B., Irimia, D. (2015) Whole blood human neutrophil trafficking in a microfluidic model of infection and inflammation. Lab Chip 15, 2625–2633. Reprinted with permission from the Centre National de la Recherche Scientifique (CNRS) and The Royal Society of Chemistry.]