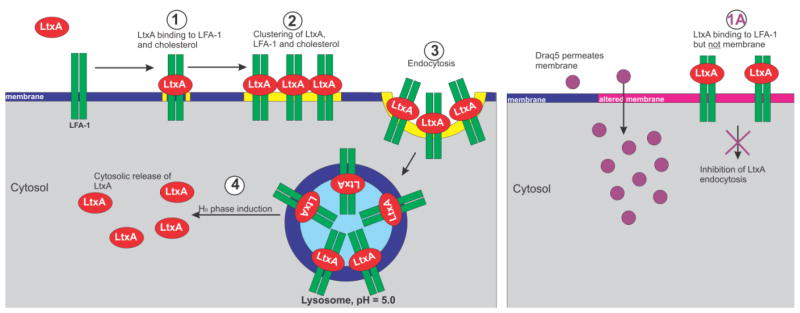
Fig. 5. Proposed mechanism of DRAQ5™-mediated inhibition of LtxA internalization.
In the absence of DRAQ5™, LtxA binds to the plasma membrane, specifically cholesterol and LFA-1 (1). This causes the formation of large-scale clusters containing LtxA, LFA-1, and cholesterol (2). LtxA is then endocytosed and localized to lysosomes (3). LtxA disrupts the lysosomal membrane (HII phase induction), to be released to the cytosol (4). In the presence of DRAQ5™, the membrane structure is altered, which prevents LtxA from binding to the membrane lipids, but does not affect the toxin’s affinity for LFA-1. This inhibition of toxin binding prevents the necessary interactions of LtxA with the membrane that promote endocytosis