Abstract
Catestatin (CST), an endogenous anti-hypertensive/anti-adrenergic peptide, is a novel regulator of cardiovascular physiology. Here, we report case-control studies in two geographically/ethnically-distinct Indian populations (n≈4000) that showed association of the naturally-occurring human CST-Gly364Ser variant with increased risk for hypertension (age-adjusted odds ratios: 1.483, p=0.009 and 2.951, p=0.005). Consistently, 364Ser allele carriers displayed elevated systolic (up to ~8 mmHg, p=0.004) and diastolic (up to ~6 mmHg, p=0.001) blood pressure. The variant allele was also found to be in linkage disequilibrium with other functional SNPs in the CHGA promoter and nearby coding region. Functional characterization of the Gly364Ser variant was carried out using cellular/molecular biological experiments (viz. peptide-receptor binding assays, nitric oxide [NO], phospho extracellular regulated kinase [ERK] and phospho endothelial nitric oxide synthase [eNOS] estimations) and computational approaches (molecular dynamics simulations for structural analysis of wild-type [CST-WT] and variant [CST-364Ser] peptides, and docking of peptide/ligand with beta-adrenergic receptors [ADRB1/2]). CST-WT and CST-364Ser peptides differed profoundly in their secondary structures and showed differential interactions with ADRB2; while CST-WT displaced the ligand bound to ADRB2, CST-364Ser failed to do the same. Furthermore, CST-WT significantly inhibited ADRB2-stimulated ERK activation suggesting an antagonistic role on ADRB2 unlike CST-364Ser. Consequently, CST-WT was more potent in NO production in human-umbilical-vein-endothelial-cells as compared to CST-364Ser. This NO producing ability of CST-WT was abrogated by ADRB2 antagonist ICI 118551. In conclusion, CST-364Ser allele enhanced the risk for hypertension in human populations, possibly via diminished endothelial NO production due to altered interactions of CST-364Ser peptide with ADRB2 as compared to CST-WT.
Keywords: genetic variation, hypertension, nitric oxide, beta-adrenergic receptor, genetic association study, chromogranin A
Chromogranin A (CHGA) is a ~50-kDa soluble, acidic glycoprotein that plays an essential role in the formation of catecholamine secretory vesicles in neuronal, endocrine and neuroendocrine tissues1. Expression levels of CHGA have been found to be elevated in rodent models of both genetic2, and acquired forms of hypertension3. Elevated plasma CHGA levels are associated with clinical severity and serve as independent prognostic indicators in patients with complicated myocardial infarction4, acute coronary syndromes5, and chronic heart failure6.
CHGA also acts as a pro-hormone and gets cleaved to give rise to several bioactive peptides7, including vasostatin (human CHGA1–76, a vasodilator and suppressor of inotropy/lusitropy)8, pancreastatin (PST; human CHGA250–301, a dysglycemic hormone)9, catestatin (CST; human CHGA352–372, an anti-hypertensive and cardio-suppressive agent), parathyroid hormone release inhibitor parastatin (human CHGA356–428)10, and serpinin (human CHGA411–436, a myocardial beta-adrenergic-like agonist)11. CST was discovered initially as a physiological brake of the adreno-sympathetic-chromaffin system due to its potent catecholamine release-inhibitory function12, 13, which it manifests by acting specifically on the neuronal nicotinic acetylcholine receptor14, 15. Plasma CST level is diminished in hypertensive individuals and even in the normotensive offspring of the established hypertensive patients, suggesting its pathogenic role in development of hypertension16. Consistently, severe hypertension in CHGA knockout (and thereby, CST-lacking) mice is rescued by the exogenous administration of CST, revalidating its role as an anti-hypertensive molecule17. Many functionally active DNA variants have been discovered in the promoter, coding and 3′-untranslated regions of the human CHGA gene7, 18. Re-sequencing of the CST expressing region of CHGA in several human populations has revealed the occurrence of a number of single nucleotide polymorphisms (SNPs) (Supp. Table S1). A previous report from our laboratory has confirmed the presence of the Gly364Ser (rs9658667) variation and, in addition, discovered a novel SNP, Gly367Val (rs200576557) in a Chennai (South India) population19. In this report, we analyzed the effect of the Gly364Ser variation on metabolic/cardiovascular disease states in a larger sample size (n=3200 individuals) in the Chennai population. As part of a replication study, we also genotyped the variant in a geographically/ethnically distinct North Indian population from Chandigarh (n=760 individuals). In both the populations, the 364Ser allele showed strong associations with elevated blood pressure (BP) levels and hypertension.
CST peptides have been found to dose-dependently reduce the effect of beta-adrenergic stimulation20. This reduction is mediated by a nitric oxide (NO) releasing action of CST in endocardial endothelial cells, rather than a direct myocardial action of the peptide. Studies in the ex vivo models of Langendorff-perfused rat heart21, amphibian (Rana esculenta) heart22, and fish (Anguilla anguilla) heart23, have also documented the anti-adrenergic as well as cardiac inotropy/lusitropy modulatory effects of CST. Based on these observations, we questioned whether regulation of NO generation by CST peptides is due to their direct interactions/effects on beta-adrenergic receptors (ADRB1/2). To understand the mechanistic basis of differential BP manifestations in the individuals due to CST peptides, we performed biochemical studies to assess NO generation, extracellular regulated kinase [ERK] activation, endothelial nitric oxide synthase [eNOS] phosphorylation and the direct binding of CST peptides to ADRB1/2. In addition, we used a comprehensive set of computational tools including molecular modelling, docking and molecular dynamics simulations to analyse the potential of CST-WT and CST-364Ser peptides to bind to ADRB1/2. CST-364Ser peptide exerted altered interactions with ADRB2 and led to diminished endothelial NO production (as compared to the CST-WT peptide) which may account for the increased risk for hypertension in 364Ser carriers.
METHODS
The detailed methodologies are included in the Supplementary Information.
Human subjects and study design
The present case-control study recruited 3200 and 760 unrelated human volunteers in Chennai (South India) and Chandigarh (North India), respectively. The detailed demographic and clinical parameters are given in the Supp. Tables S2 and S3.
Each subject gave informed, written consent for the use of their blood samples for genetic and biochemical analyses in this study. This study was approved by the Institute Ethics Committee at IITM in accordance with Declaration of Helsinki (reference number: IITM IEC No. 2007008).
The exon-7 region of CHGA was re-sequenced in 1763 subjects to detect the presence of SNPs in the CST, PST and parastatin domains. Another 2197 subjects were genotyped for the Gly364Ser SNP by Taqman® allelic discrimination method. We also re-sequenced the CHGA promoter region in 581 study subjects using specific primers19.
Data representation and statistical analysis
The experimental data results and the phenotypic characteristics in the human study are expressed as mean ± SE. Allele frequencies were estimated by gene counting. A Pearson’s χ2 test was employed to compare the distribution of the genotypes. Statistical analysis was carried out using the Statistical Package for Social Sciences (SPSS) version 21.0. Haploview 4.2 was used for linkage disequilibrium (LD) analysis24. A p value of <0.05 was chosen as statistically significant. The power of the study was calculated using Quanto version 1.2.4.25. Meta-analysis was carried out using the OpenMeta[Analyst] software (www.cebm.brown.edu/open_meta/).
Synthesis of CST Peptides
The CST wild-type (CST-WT, SSMKLSFRARAYGFRGPGPQL) and CST-364Ser variant (CST-364Ser, SSMKLSFRARAYSFRGPGPQL) peptides were synthesized by solid phase method and purified as described previously19.
Measurement of NO levels and eNOS activity in cultured human umbilical vein endothelial cells (HUVECs)
Experimental procedures involving umbilical cords were reviewed and approved by the IIT Madras institutional ethics committee in accordance with Declaration of Helsinki revised in 2000 (reference number: IITM IEC No. 2009024). HUVECs were isolated from umbilical cords by digestion with collagenase as described previously26. NO levels in HUVECs were measured by 4, 5-Diaminofluorescein diacetate (DAF-2 DA) method as described previously27. Activation of eNOS in HUVECs by CST peptides was assessed by Western blotting and detection of phospho-eNOS-Ser1177.
Isolation of ADRB1/2-expressing plasma membranes, radio-ligand binding and competition binding assays
HEK-293 cells stably expressing ADRB1/2 were treated with isoproterenol and CST peptides. Activation of ERK as a measure of ADRB1/2-activation in ADRB1/2 HEK-293 cells was assessed by immunoblotting and detection of phospho-ERK as described previously28.
Purification of plasma membranes from control HEK-293 and ADRB1/2 HEK-293 cells was performed as described previously29, 30. To test the level of ADRB1/2 expression, [125I]-cyanopindolol saturation radio-ligand binding was performed on the isolated plasma membranes. Competition binding was performed by incubating 20 μg of plasma membranes with saturating concentrations of CST-WT and CST-364Ser peptides in the range of 10 pmol/L to 1 mmol/L.
Homology modeling of ADRB1/2 receptors and CST peptides and analysis of peptide-receptor interactions
The crystal structure of ADRB2 with resolution 2.4 Å, was obtained from protein data bank (PDB ID:2RH1)31. The structure of ADRB1 was modelled by using the structure of ADRB2 as a template. The 3D structures of CST-WT and CST-364Ser were generated following a similar protocol as proposed earlier32. The residue Gly364 in the NMR structure of CST (PDB ID:1LV4) was mutated to 364Ser using Modeller 9v1333. Short energy minimizations were performed on both the peptide structures to optimize the side chain positions. The minimized structures were subsequently subjected to 300 nanoseconds explicit water molecular dynamics (MD) simulations to generate an ensemble of refined CST-WT and CST-364Ser conformations.
Protein-protein dockings of CST peptides on ADRB1/2 were performed using ZDOCK algorithm34. During molecular docking, CST peptides were allowed to search the extracellular region of the ADRB1/2 receptors to identify the best binding location. Out of the 100 binding modes of CST peptides to ADRB1/2, the best docked complex was identified based on the ZDOCK score.
All the structural figures were rendered using Visual Molecular Dynamics (VMD)35. The CST-ADRB2 interactions were identified using PDBsum36, and cyanopindolol-ADRB2 interactions were identified using LigPlot+37.
RESULTS
Discovery and occurrence of the CST Gly364Ser SNP in Indian populations
Re-sequencing of the CST region of CHGA in 1763 subjects from an urban Chennai (South Indian) population consisting of type 2 diabetes (DM) / hypertension (HTN) cases and controls led to discovery of two variants: Gly364Ser (rs9658667) and Gly367Val (rs200576557). Because the Gly364Ser variation was common (>5% minor allele frequency [MAF]), we genotyped additional 918 subjects for this SNP by Taqman® allele discrimination method in the same population. We also genotyped the SNP in a population of Coronary Artery Disease (CAD) patients from the same region (Chennai). The Gly364Ser SNP, which is caused by an A to G transition at the 9559 bp position leading to the substitution of codon GGC by codon AGC at the 364th amino acid position of the mature CHGA protein (Supp. Fig. S1), was found to occur at 6.34% MAF, i.e., in ~13% of the study population (Supp. Table S4). We then carried out a second replication study in a population from Chandigarh (North Indian) consisting of hypertensive cases and controls. Here, surprisingly, we found the SNP at a much lower MAF (3.48%), i.e., only in ~7% of the population, without the presence of a single homozygous variant in 760 individuals (Supp. Table S4).
Genotype frequencies were found to be in Hardy-Weinberg Equilibrium (HWE) in the Chennai DM/HTN (χ2=1.286, p=0.256), Chennai CAD (χ2=0.250, p=0.617), Chandigarh HTN (χ2=1.047, p=0.306) and Chandigarh controls (χ2=0.142, p=0.706) populations. The Chennai controls population, however, showed a departure from HWE (χ2=6.799, p=0.009). On closer observation of the genotypes in this population, we found that the presence of only two homozygous variants accounted for this departure.
364Ser allele is associated with hypertension in independent populations
The Chennai population was divided into different disease groups (DM, HTN and CAD) and logistic regression analysis was carried under both the genotype (GG vs. AG and GG vs. AA) and dominant (GG vs. AG+AA) genetic models. Due to the small number of homozygous-variant individuals (n=18), the recessive model was not used. Gly364Ser allele was found to be associated with hypertension under both the models, with unadjusted odds ratios (OR) of 1.440 (95% confidence interval [CI]=1.072–1.933, p=0.015) for GG vs. AG and 1.385 (CI=1.039–1.846, p=0.026) for GG vs. AG+AA (Table 1). The associations retained significance even after adjusting individually for age, sex, body mass index (BMI) as well as all three factors together (Table 1). An additional adjustment for anti-hypertensive medications along with age, sex and BMI also showed significant association for GG vs. AG+AA at OR=1.694 (CI=1.018–2.819, p=0.042). Interestingly, stronger associations of the 364Ser allele with hypertension were detected in a subgroup (having BMI <24) of this population under both dominant genetic model (unadjusted OR=1.856, CI=1.234–2.792, p=0.003; age-adjusted OR=1.983, CI=1.312–2.997, p=0.001; sex-adjusted OR=1.856, CI=1.227–2.809, p=0.003) and GG vs. AG genotype model (unadjusted OR=1.754, CI=1.155–2.662, p=0.008; age-adjusted OR=1.854, CI=1.216–2.826, p=0.004; sex-adjusted OR=1.775, CI=1.164–2.709, p=0.008). The 364Ser allele also showed higher frequency in subjects having any of the three disease states (DM/HTN/CAD); although the unadjusted ORs were not significant, adjustment with age yielded modestly significant ORs of 1.325 (CI=1.024–1.714, p=0.032) and 1.299 (CI=1.011–1.667, p=0.041) under the genotype and dominant models, respectively.
Table 1.
Association of CST 364Ser allele with risk for hypertension.*
| Population | Genotype | OR (95% CI); p value
|
||||
|---|---|---|---|---|---|---|
| Unadjusted | Age adjusted | Sex adjusted | BMI adjusted | Age, Sex and BMI adjusted | ||
| Chennai | GG vs. AG | 1.440 (1.072–1.933); p=0.015 | 1.483 (1.103–1.994); p=0.009 | 1.431 (1.063–1.926); p=0.018 | 1.441 (1.072–1.938); p=0.015 | 1.469 (1.087–1.984); p=0.012 |
| Chennai | GG vs. AG+AA | 1.385 (1.039–1.846); p=0.026 | 1.429 (1.070–1.907); p=0.015 | 1.380 (1.033–1.845); p=0.030 | 1.393 (1.043–1.858); p=0.025 | 1.424 (1.062–1.909); p=0.018 |
| Chandigarh | GG vs. AG | 2.662 (1.420–4.990); p=0.002 | 2.951 (1.390–6.265); p=0.005 | 2.639 (1.389–5.013); p=0.003 | n.c. | n.c. |
Logistic regression analysis was carried out in the Chennai and Chandigarh populations. Odds ratio for hypertension (HTN) was analysed. The analyses were done both by the genotype (GG vs. AG and GG vs. AA) as well as dominant (GG vs. AG+AA) genetic models for the Chennai population. n.c.: not calculable due to unavailability of BMI data in this study population.
The replication population (Chandigarh) also showed strong association of the 364Ser allele with hypertension. Since there were no homozygous variant individuals identified in this population, the logistic regression analysis based on both genotype and dominant models yielded the same results. The unadjusted OR was highly significant at 2.662 (CI=1.420–4.990, p=0.002) (Table 1). The association persisted even after adjusting for age at OR=2.951 (CI=1.390–6.265, p=0.005), sex at OR=2.639 (CI=1.389–5.013, p=0.003) and age & sex together at OR=2.862 (CI=1.359–6.028, p=0.006) (Table 1). Adjusting for smoking and dyslipidemia as well (data for which was not available for a large section of our study subjects) would have made this study stronger.
Association of 364Ser variant with elevated BP levels in independent populations
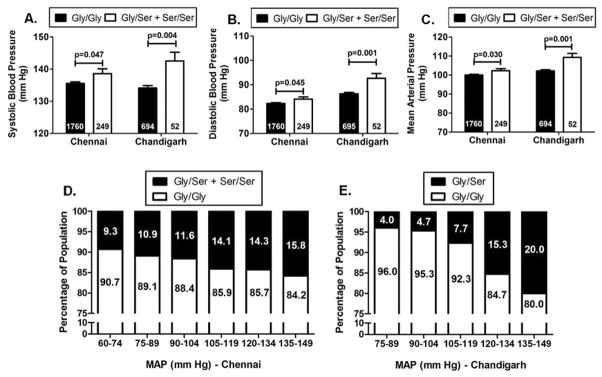
In the primary Chennai population, there was a significant trend of increased BP levels in the carriers of the 364Ser allele as compared to the wild-type individuals. Initially, we compared the BP levels among the different genotype groups in 2069 individuals from the overall DM/HTN population. The variant individuals had ~2.5 mmHg higher systolic blood pressure (SBP, p=0.045), ~1.5 mmHg higher diastolic blood pressure (DBP, p=0.074) and ~2 mmHg higher mean arterial pressure (MAP, p=0.043) levels than the wild-type individuals. Next, to adjust for the effect of the anti-hypertensive medications, 60 individuals without information for anti-hypertensive medication were removed and the analysis was repeated after adjusting BP for medication in the remaining 2009 individuals38. After drug adjustment, 364Ser carriers displayed ~3 mmHg higher SBP (p=0.047), ~2 mmHg higher DBP (p=0.045) and ~2.5 mmHg higher MAP (p=0.030) levels as compared to Gly364 carriers (Fig. 1A, 1B, 1C). Adjusting for age via ANCOVA further strengthened the association for SBP (p=0.031), DBP (p=0.044) and MAP (p=0.025) (Table 2).
Figure 1. Allele-specific associations of the CST Gly364Ser variation with blood pressure.
Panels A, B and C: Data are shown as mean ± SE. SBP (A), DBP (B) and MAP (C) levels in the carriers of 364Ser allele were higher (analyzed by independent samples t-test using SPSS version 21.0) than the wild-type individuals in the overall Chennai and Chandigarh populations.
Panels D and E: Data are shown as percentage. The percentage of individuals harboring the 364Ser allele showed an increase with increase in the range of the MAP levels in both the Chennai (D) and Chandigarh (E) populations.
Table 2.
Meta-analysis of the 364Ser allele effects on blood pressure in Asian populations.*
| Population | Parameter | Gly/Gly
|
Gly/Ser + Ser/Ser
|
Effect size | 95% Confidence Interval
|
Unadjusted p value | Adjusted p value (ANCOVA) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean | SEM | N | Mean | SEM | Lower Boundary | Upper Boundary | |||||
| Chennai/South Indian | SBP | 1760 | 135.50 | 0.54 | 249 | 138.57 | 1.52 | 3.07 | −0.09 | 6.23 | 0.047 | 0.031 |
| DBP | 1760 | 82.33 | 0.31 | 249 | 84.12 | 0.84 | 1.79 | 0.03 | 3.55 | 0.045 | 0.044 | |
| MAP | 1760 | 100.04 | 0.37 | 249 | 102.3 | 1.00 | 2.26 | 0.17 | 4.35 | 0.030 | 0.025 | |
| Chandigarh/North Indian | SBP | 694 | 134.12 | 0.76 | 52 | 142.54 | 2.70 | 8.42 | 2.92 | 13.92 | 0.004 | 0.009 |
| DBP | 695 | 86.33 | 0.49 | 52 | 92.65 | 2.00 | 6.32 | 2.28 | 10.36 | 0.001 | 0.004 | |
| MAP | 694 | 102.24 | 0.55 | 52 | 109.29 | 2.07 | 7.05 | 2.85 | 11.25 | 0.001 | 0.003 | |
| Ibaraki, Saitama, Shizuoka/Japanese | SBP | 301 | 132.00 | 1.14 | 42 | 138.20 | 2.72 | 6.20 | 0.42 | 11.98 | 0.055 | 0.048 |
| DBP | 301 | 80.30 | 0.60 | 42 | 82.00 | 1.37 | 1.70 | −1.23 | 4.63 | 0.314 | n.s. | |
| MAP | 301 | 100.7 | 0.86 | 42 | 104.50 | 1.97 | 3.80 | −0.41 | 8.01 | 0.117 | n.s. | |
| PP | 301 | 51.70 | 0.72 | 42 | 56.10 | 1.92 | 4.40 | 0.382 | 8.418 | 0.030 | 0.025 | |
| Overall Population | SBP | 2755 | 343 | 5.21 | 1.92 | 8.50 | <0.01 | |||||
| DBP | 2756 | 343 | 2.76 | 0.40 | 5.11 | 0.02 | ||||||
| MAP | 2755 | 343 | 3.93 | 1.12 | 6.73 | <0.01 | ||||||
Meta-analysis was carried out using the data for the effect size of the Gly364Ser SNP in the three Asian populations of Chennai, Chandigarh and Japan. The data for the Japanese population was obtained from Choi et. al59. Age-adjusted ANCOVA was carried out in the Chennai and Chandigarh populations. For the Japanese population, the ANCOVA was carried out considering gender, age, BMI, anti-hypertensive medication, diabetes, dyslipidemia and smoking as covariates. SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; PP, pulse pressure calculated from SBP and DBP. The three independent populations displayed directionally-concordant effect on blood pressure. Meta-analysis results show an overall significant effect of elevated blood pressure in 364Ser allele carriers. n.s., not significant.
In the Chandigarh population as well, we found 364Ser to be associated with elevated BP levels. The variant allele carrying individuals showed ~8 mmHg higher SBP (p=0.004), ~6 mmHg higher DBP (p=0.001) and ~7 mmHg higher MAP (p=0.001) levels than the wild-type individuals (Fig. 1A, 1B, 1C). For the hypertensive cases, the pre-treatment BP levels were considered for association.
We further divided our Chennai and Chandigarh populations into different BP ranges and calculated the frequencies of the 364Ser allele in each range. With an increase in the severity of the disease there was an increase in the percentage of people harboring the variant allele (for Chennai: linear-by-linear association χ2=3.99 and p=0.046, for Chandigarh: linear-by-linear association χ2=12.89 and p=0.0003) (Fig. 1D, 1E).
The unadjusted power of the study for the hypertensive Chennai population was 65.6% and on adjusting for age, sex and BMI, the power of the study stood at 73.1%. For the Chandigarh population, the unadjusted power was 98.5%, while power adjusted for age and sex was 98.3%.
364Ser allele is in linkage disequilibrium (LD) with CHGA promoter SNPs and neighboring exon-7 coding variants
Previous studies have discovered the occurrence of common SNPs in the CHGA promoter as well as coding regions18, 19, 39. Many of these SNPs have been found to be functionally active, either in altering the transcriptional activity of the promoter18 or the potencies of the peptides they are found in18, 32, 39, 40. We had the complete genotyped data for 581 individuals for the Gly364Ser SNP, 8 CHGA promoter SNPs [−1106G→A (rs9658628), −1018A→T (rs9658629), −1014T→C (rs9658630), −988T→G (rs9658631), −462G→A (rs9658634), −415T→C (rs9658635), −89C→A (rs7159323), −57C→T (rs9658638)] and 3 other neighboring SNPs in the CHGA exon-7: Gly297Ser (rs9658664), Arg381Trp (rs729940), Glu403Glu (rs729939). To test whether the Gly364Ser variant is likely to be segregated with any of these other common SNPs, and whether association with the variant is also being contributed by them, we carried out an LD analysis using the genotyped data for the 581 subjects. The Gly364Ser variant was found to be in LD with 7 out of the 11 polymorphisms: 4 in promoter (−1106G→A, −1018A→T, −415T→C and −57C→T) and 3 in CHGA exon-7 (Gly297Ser, Arg381Trp and Glu403Glu) (Supp. Fig. S2).
CST peptides differ in nitric oxide (NO) production ability in human umbilical vein endothelial cells (HUVECs)
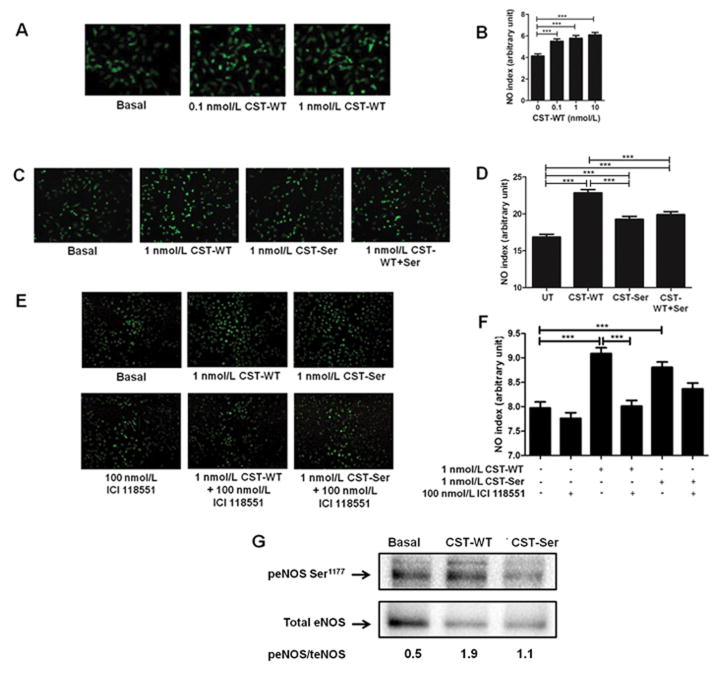
In order to investigate the increase in BP levels in the presence of the 364Ser allele, we tested whether the wild-type and variant peptides differ in their efficacies in inducing NO production in vascular endothelial cells. Initially, we treated HUVECs with two doses of CST-WT (0.1 nmol/L and 1 nmol/L), both of which significantly increased (p<0.001) the NO levels as compared to the basal condition (Fig. 2A). The NO indices followed the order: 1 nmol/L CST-WT (2.50) > 0.1 nmol/L CST-WT (2.35) > basal (1.90) (Fig. 2B). Since at both the doses the peptide showed significant effect, we then chose to continue with a dose of 1 nmol/L to compare the activities of the variant and the wild-type peptide. Treatment of HUVECs with CST-WT or CST-364Ser showed that NO indices were increased with both the peptides compared to basal (p<0.001), but however, the increase with CST-WT was significantly higher than CST-364Ser variant (p<0.001) (Fig. 2C); the NO indices in HUVECs were in the following order: CST-WT (22.85) > CST-364Ser (19.26) > basal (16.85) (Fig. 2D). Interestingly, when both the peptides were added in an equimolar ratio as a representative of the heterozygous condition, the NO index (19.89) was in between that of the wild-type and variant peptide (Fig. 2C and 2D).
Figure 2. Effect of CST peptides on NO production in HUVECs.
The fluorescence intensities (NO indices) were calculated by Image J analysis and plotted as mean ± SE. The experimental groups were compared by one-way ANOVA followed by Tukey’s multiple comparison post-test.
Panels A and B: Representative images for the treatment of HUVECs with different doses of CST-WT (0.1 nmol/L and 1 nmol/L). ***p<0.001; one-way ANOVA F=15.71, p<0.0001, n=50 cells/condition.
Panels C and D: Representative images for the treatment of HUVECs with CST peptides. ***p<0.001; one-way ANOVA F=37.15, p<0.0001, n=450 cells/condition. The order for efficacy of peptides in NO production: CST-WT>CST-WT+Ser>CST-364Ser>basal.
Panels E and F: Representative images for the treatment of HUVECs with CST peptides and ADRB2 antagonist ICI 118551. ***p<0.001; one-way ANOVA F=19.65, p<0.0001, n=450 cells/condition.
Panel G: Representative western blot of 3 independent experiments showing phosphorylated Ser1177-eNOS (peNOS) and total eNOS (teNOS) levels upon treatment of HUVECs with CST peptides. The peNOS/teNOS values have been indicated below each lane.
In view of the direct links between stimulation of cardiovascular beta-adrenergic receptors and NO generation41, we sought to test whether the NO effects of the CST peptides are routed through their interactions with the beta-adrenergic receptors ADRB1 and ADRB2. Accordingly, we treated HUVECs with ADRB1/2 antagonists (viz. CGP 20712 for ADRB1 and ICI 118551 for ADRB2), followed by treatment with the CST peptides. The ADRB2 antagonist was found to significantly blunt the NO increasing effect of the CST-WT peptide (NO index for CST-WT: 9.09 vs. NO index for CST-WT+ICI 118551: 8.01) while it did not do the same in the case of CST-364Ser peptide (NO index for CST-364Ser: 8.80 vs. NO index for CST-364Ser+ICI 118551: 8.36) (Fig. 2E and 2F). ADRB1 antagonist failed to show any inhibition of the NO levels produced by both peptides (Supp. Fig. S3).
Next, to assess the effect of CST peptides on eNOS activity, we checked the phosphorylation levels of eNOS at Ser1177 residue, after treatment with the CST peptides. CST-WT increased the phosphorylation at Ser1177 of eNOS as compared to CST-364Ser (Fig. 2G). Since phosphorylation at Ser1177 leads to activation of eNOS, these results suggest an overall higher eNOS activity in cells treated with CST-WT.
Differential interactions of CST peptides with beta-adrenergic receptors (ADRB1/2): experimental evidence
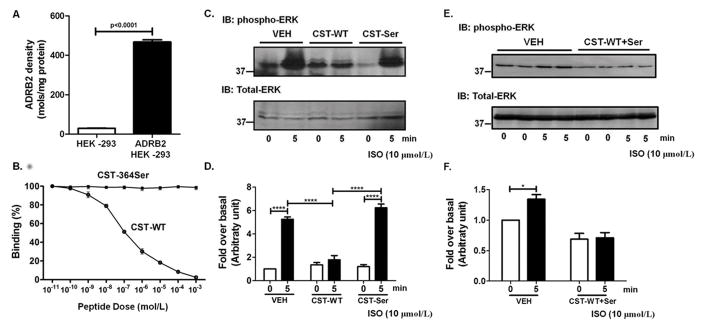
We then tested the interactions of the CST peptides with ADRB1/2 to see whether their altered interactions with either of the receptors can explain their differential NO effects. Competition binding assays were performed with [125I]-cyanopindolol using HEK-293 cells stably expressing human ADRB1/2. The levels of ADRB1/2 expression were assessed by performing radio-ligand binding using saturating concentrations of [125I]-cyanopindolol on plasma membranes isolated from ADRB1/2 expressing HEK-293 cells. ADRB1 HEK-293 cells showed ~109-fold (p<0.0001) higher levels of ADRB1 expression (Supp. Fig. S4A) as HEK-293 cells have very sparse endogenous expression of ADRB1. ADRB2 HEK-293 cells showed ~16-fold (p<0.0001) higher level of ADRB2 expression compared to control HEK-293 cells (Fig. 3A) as HEK-293 cells do have some level of endogenous ADRB2s. Plasma membranes from ADRB1/2 HEK-293 cells were then subjected to an indirect competition ligand binding assay wherein we tested for the ability of increasing doses (10 pmol/L to 1 mmol/L) of CST-WT or CST-364Ser peptides to displace saturating concentrations of labelled cyanopindolol. In ADRB2 HEK-293 cells, CST-WT peptide competitively displaced the [125I]-cyanopindolol with increasing concentrations (p<0.0001, F=2300, R2=0.998) in contrast to CST-364Ser peptide which did not displace or compete with cyanopindolol (Fig. 3B). However, in ADRB1 HEK-293 cells, both the peptides failed to displace [125I]-cyanopindolol with increasing concentrations (Supp. Fig. S4B).
Figure 3. Binding of CST peptides to ADRB2 receptor and downstream effects.
Panel A: ADRB2 HEK-293 cells showed ~16-fold higher expression of ADRB2 (p<0.0001 by 2-tailed unpaired t-test) as compared to control HEK-293 cells. Data are shown as ADRB2 levels normalized with total protein.
Panel B: Data are shown as percentage binding of the radio-ligand cyanopindolol. With increasing doses of CST-WT (10 pmol/L to 1 mmol/L) the ligand got completely displaced (p<0.0001, F=2300, R2=0.998) while with increasing doses of CST-364Ser, there was no effect. The experimental groups were compared by one-way ANOVA followed by Tukey’s multiple comparison post-test.
Panels C and D: Representative western blot (C) and quantitative representation of the densitometric analysis from 4–6 independent experiments (D) showing phosphorylated ERK (pERK) and total ERK levels upon treatment with CST peptides and isoproterenol (ISO). ISO (10 μmol/L) showed an increase in pERK levels at 5 min in the vehicle (VEH) condition, reflecting the activation of ADRB2. However, this increase was inhibited upon pre-treatment with CST-WT (10 μmol/L). ****p<0.0001. On the other hand, pre-treatment with CST-364Ser (10 μmol/L) showed levels of activation similar to the vehicle. The experimental groups were compared by 2-tailed t-test.
Panels E and F: Representative western blot (E) and quantitative representation of the densitometric analysis from 4 independent experiments (F) showing phosphorylated ERK (pERK) and total ERK levels upon treatment with equimolar ratios of CST-WT and CST-364Ser peptides and isoproterenol (ISO). ISO (10 μmol/L) showed an increase in pERK levels at 5 min in the vehicle (VEH) condition. However, this increase was inhibited upon pre-treatment with CST-WT+Ser (10 μmol/L). *p<0.05. The experimental groups were compared by 2-tailed t-test.
To further check whether binding of these peptides has consequences in beta-adrenergic signalling, receptor activation was assessed by determining the phosphorylation status of extra-cellular regulated kinase (ERK). ADRB1/2 HEK-293 cells were pre-treated with either CST-WT or CST-364Ser peptide followed by ADRB agonist isoproterenol stimulation. While in ADRB2 HEK-293 cells, pre-treatment with CST-WT inhibited ADRB2-mediated ERK activation (p<0.0001) CST-364Ser had no appreciable effects in altering ERK (Fig. 3C and 3D). Interestingly, treatment with equimolar ratios of CST-WT and CST-364Ser elicited a similar response to that of CST-WT alone (Fig. 3E and 3F). In ADRB1 HEK-293 cells, on the other hand, both the peptides failed to show any detectable effects in altering ERK levels (Supp. Fig. S4C and S4D). These studies suggest that CST-WT may be acting as an inhibitor/antagonist to ADRB2 function in contrast to CST-364Ser which does not alter the ADRB2 function. Moreover, this differential effect seems to be limited to the ADRB2 receptor only and not the ADRB1 isoform.
Structures of the CST peptides and their differential interactions with beta-adrenergic receptors (ADRB1/2): computational analysis
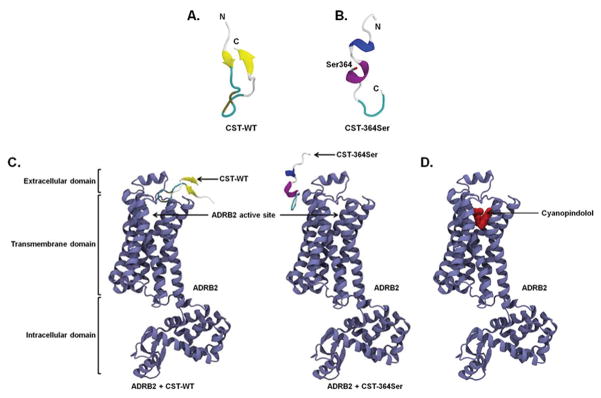
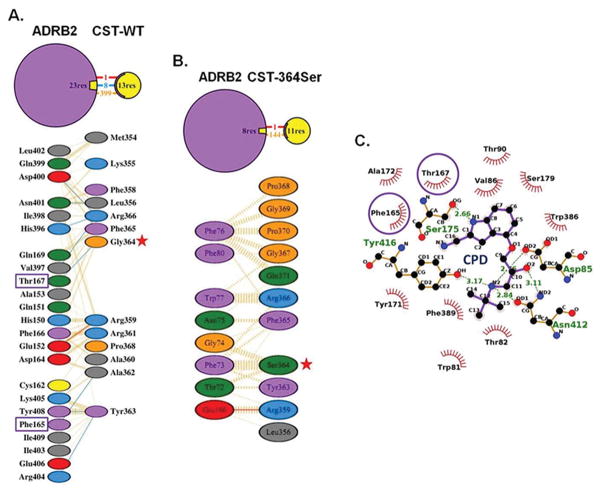
In order to explore whether the differential effects of the peptides in vitro can be attributed to any structural differences between them we generated in silico models of CST peptides and CST-ADRB1/2 complexes, and performed structural analysis on them using protein-protein modeling and molecular dynamics (MD) simulations (Fig. 4). The CST-WT structure was found to comprise of a metastable anti-parallel β-sheet and a random coil (Fig. 4A). Its N-terminal β-strand was stabilized by interactions between Lys355, Leu356 and Ser357, whereas the C-terminal β-strand was stabilized by Gly369, Pro370 and Gln371. Interestingly, the mutation of Gly364 to 364Ser drastically changed the secondary structural content of CST. CST-364Ser displayed a metastable 310 helix and a stable α-helix in the central region (Fig. 4B). The 310 helix was stabilized by residues Leu356, Ser357 and Phe358 and the stable α-helix was comprised of Arg361, Ala362, Tyr363, and Ser364. All residues of the time-averaged structures of both peptides from simulations are in the allowed regions of the Ramachandran plot (Supp. Fig. S5), thus validating the proposed models of the peptides.
Figure 4. Structures of CST peptides and complexes of CST peptides/cyanopindolol with ADRB2 receptor.
The time averaged structures of CST-WT (A) and CST-364Ser (B) are shown in cartoon representation. The 364Ser mutation in the CST-364Ser peptide is shown in a stick representation. (C) Snapshots of CST-WT (left) and CST-364Ser (right) docked to ADRB2. ADRB2: violet, beta-sheet in CST-WT: yellow, alpha-helix in CST-364Ser: purple and 310-helix in CST-364Ser: blue. (D) Snapshot of the docked complex of cyanopindolol (red, van der Waals representation) and ADRB2 (violet, cartoon representation).
The binding of CST peptides to ADRB1/2 was explored via protein-protein docking in which both CST-WT and CST-364Ser were allowed to sample the extracellular region of the modeled ADRB1/2 (Supp. Fig. S6) independently. In case of ADRB1, both the CST peptides did not show any significant binding to the ADRB1 active site (Supp. Fig. S7). On the other hand, in case of ADRB2, they were found to bind to different locations of the receptor (Fig. 4C). While the “thumb-like” structure of CST-WT could fit into the ligand entry site of ADRB2 due to its shape complementarity, CST-364Ser failed to dock into the ligand entry site due to its linear stretched structure and difference in secondary structural content compared to CST-WT. It instead bound to the outer surface of the receptor, away from the CST-WT binding site. A brief 50 ns MD simulation was performed on each of these CST-ADRB2 complexes in lipid bilayer for further refinement, but no significant change in binding mode was observed. The ZDOCK score (calculated based on surface complementarity, electrostatistics, and statistics potential) was 1197 for CST-WT & ADRB2 and 1067 for CST-364Ser & ADRB2, implying better binding of CST-WT to ADRB2.
To reconfirm the binding of CST-WT to ADRB2, the high-affinity ligand cyanopindolol was docked to the peptide-receptor complexes. In >100 attempts for protein-ligand docking through AutoDock42, cyanopindolol could never bind to the CST-WT-ADRB2 complex, while it bound effectively to the CST-364Ser-ADRB2 complex in all the attempts. This further proves the complete occlusion of the receptor’s ligand binding pocket by CST-WT and out-of-pocket binding of CST-364Ser to ADRB2 (Fig. 4C).
To check the competitive binding of CST-WT and cyanopindolol to ADRB2, we produced the cyanopindolol-ADRB2 complex by protein-ligand docking (Fig. 4D). Very similar to the available crystal structure of cyanopindolol-ADRB1 complex (PDB ID: 4BVN)43, cyanopindolol was found to bind to the hollow region of ADRB2 formed by the seven transmembrane helices. A closer look at the interactions involved in the CST-WT-ADRB2 and cyanopindolol-ADRB2 complex formation revealed that even though cyanopindolol binds deep into the pocket, there were two common ADRB2 residues (viz. Phe165 and Thr167) which interacted with both CST-WT (Fig. 5A) and cyanopindolol (Fig. 5C). Of note, ADRB2:Thr167 interacts with CST-WT:Gly364 strongly through hydrogen bonds during the CST-WT-ADRB2 complex formation, thus, making CST-WT:Gly364 a crucial residue for complex formation. Therefore, it is not surprising that a mutation at this particular residue of CST makes its binding to ADRB2 active site weaker or there is no binding. The interactions in the CST-364Ser-ADRB2 complex formation involve 0 hydrogen bonds (in contrast to 8 in CST-WT-ADRB2 complex) and 1 salt bridge. The remaining interactions are hydrophobic in nature, including the ones involving 364Ser, making this binding a very weak one (Fig. 5B). An analysis of the Gly364Ser mutation using the polyphen-2 tool (which estimates the possible impact of an amino acid substitution on the structure and function of a human protein with the help of sequence, phylogenetic and structural information characterizing the substitution) predicted this particular SNP to be ‘possibly damaging’ with a score of 0.528 (sensitivity: 0.8; specificity: 0.9)44.
Figure 5. Molecular interactions in the complexes of CST peptides or cyanopindolol with ADRB2.
Panels A and B: Binding interactions in CST-WT-ADRB2 (A) and CST-364Ser-ADRB2 (B) complexes. Hydrogen bonds: blue lines, hydrophobic contacts: orange lines and salt bridges: red lines. Each residue is color-coded based on its nature with aliphatic residues in grey; aromatic residues in pink; negatively charged residues in red; positively charged residues in cyan; neutrally charged residues in green; Pro and Gly in orange and Cys in yellow. Gly364Ser polymorphism: red stars.
Panel C: Binding interactions of cyanopindolol with ADRB2. Hydrophobic interactions: red spiked semi-circles and hydrogen bonding interactions: green dotted lines with distance values indicated.
Common interacting residues of ADRB2 with CST-WT and cyanopindolol are highlighted using purple boxes in Panel A and purple circles in Panel C, respectively.
DISCUSSION
Catestatin: a CHGA-derived anti-hypertensive peptide
Recent studies have provided ample evidence to testify CST as a multifunctional peptide with diverse roles in the regulation of cardiovascular/metabolic functions45, 46. Given that its precursor CHGA is a candidate gene for essential hypertension47, CST’s role as an anti-hypertensive agent has been an interesting topic of research. The primary evidence for this was found when the administration of exogenous CST resulted in the rescue of the hypertensive and hyperadrenergic phenotypes exhibited by CHGA knock-out mice17, 48. The role of CST as a potent vasodilator in vivo has also been well documented both in rats 49 as well as in humans50. CST also seems to be capable of modulating the components of the brainstem circuitry (rostral and caudal ventrolateral medulla) that regulate BP51, 52.
The occurrence of the Gly364Ser SNP in diverse ethnic world populations
The discovery of a functionally active variant of CST (Gly364Ser) in a Southern Californian population held promise of providing small, yet important, clues in elucidating the mechanism for the development of hypertension18. However, owing to the vast difference in the genetic make-up of the different ethnic populations across the world, it would be irrational to generalize the magnitude and direction of allelic effect sizes across populations53, 54. A study in the PAGE (Population Architecture using Genomics and Epidemiology) consortium of multi-ancestry populations has very ably demonstrated that differential LD between common polymorphisms (tagSNPs) and functional variants within diverse populations significantly contribute to diluting the effect sizes among these populations55. The immense variation in the distribution of genotypes for the Gly364Ser polymorphism in different world populations foreshadows a similar distortion in the effect sizes in these populations (Supp. Table S5). Overall, the SNP seems to be occurring in three strata of ethnic groups. The first stratum, with a high allelic frequency (6–8%), includes the Asian and Hispanic groups. The European ethnicity forms the second stratum, which has a moderate frequency (2–5%). The third stratum, with the lowest frequency (0–1%), consists of the African populations. In our previous study19, we reported the discovery of the Gly364Ser polymorphism in an urban Chennai population (n=1010 individuals) at an MAF of ~8.0%. In this study, we have expanded the sample size to 3200 individuals consisting of DM/HTN/CAD/controls from the same population. 364Ser allele displayed a MAF 6.34% in the Chennai DM/HTN/CAD population which seems to be consistent with the frequencies observed in other Asian populations. Interestingly, the ethnically-distinct Chandigarh population displayed a much lesser MAF of 3.48% which is closer to that observed in the European stratum than the Asian one. The distribution of the genotype frequencies differed significantly between Chennai DM/HTN/CAD population and Chandigarh population (χ2=18.01 and p=0.0001). Thus, CST region of CHGA seems to be displaying significant genetic variations among different world ethnic populations. In the evolutionary context, the 364Ser variant has not been detected in other mammals (Supp. Fig. S8). Some mammals (e.g. Giant panda), however, have Asp at the 364th position.
Association of the CST 364Ser allele with hypertension
The logistic regression analysis revealed a very significant association of the 364Ser allele with hypertension in both the primary (Chennai) as well as the replication (Chandigarh) populations (Table 1). This was supported by the observation of elevated BP levels in the 364Ser carriers in both the study populations. Despite the difference in the frequency of the Gly364Ser polymorphism in these two ethnically distinct populations, its effect on BP levels seems to replicate well. The higher occurrence of the variant allele in the population with an increase in BP ranges further provides the evidence of association of the 364Ser allele with hypertension (Fig. 1D and 1E). Overall, the 364Ser allele seems to act as a risk allele for hypertension development in Indian populations.
Why has Gly364Ser not been detected in the genome-wide association studies (GWAS) carried out on cardiovascular diseases till date? Firstly, most of these GWAS were carried out in either European or American populations wherein the MAFs for this SNP are much lower than those in Indian populations (Supp. Table S5). Owing to the low MAFs, very large sample sizes would be required to identify any significant association with this SNP in these populations. Secondly, almost all the genechip arrays (for example, Illumina Human550K Bead chip and Illumina Human610K Bead chip) used in these reported studies as well as other studies carried out among Asians56, 57, did not harbour the Gly364Ser SNP. Therefore, the fact that these GWAS did not identify Gly364Ser as a risk variant for hypertension is not surprising.
We found the 364Ser allele to be in LD with the CHGA promoter SNPs at −1106, −1018, −415 and −57 bp positions (Supp. Fig. S2). The 8 SNPs in the CHGA promoter form haplotypes which differ from each other in terms of their transcriptional activity18. The minor alleles at −1018, −415 and −57 bp positions of the CHGA promoter give rise to one of the 5 common CHGA promoter haplotypes (GTTTGCCT) which shows higher promoter activity as compared the wild-type consensus haplotype (GATTGTCC)18. This would mean that the 364Ser carriers may have a more active promoter leading to greater levels of the parent CHGA molecule being produced. Elevated levels of CHGA are associated with elevated BP levels58. Therefore, the hypertensive effect of the 364Ser allele might be getting manifested through increased CHGA promoter activity as well. 364Ser allele is also in modest LD with Gly297Ser, a functionally active SNP in PST that seems to alter the risk for type-2 diabetes in an Indian population32. Thus, the association of the 364Ser allele with elevated plasma glucose levels (Supp. Fig. S9) may be an effect of it being a ‘by-stander’.
Of note, in a Southern Californian population, the 364Ser allele displayed association with diminished DBP levels, especially in men; however, the effect was not consistently observed for SBP or in women39. Conversely, the 364Ser allele was associated with elevated SBP and MAP levels in a Japanese population (Table 2)59. Thus, 364Ser allele seems to exert directionally concordant effects on BP in several Asian populations (South Indian, North Indian and Japanese) although not in Caucasians. This is similar to a previous study reporting that the 12Ala allele in the peroxisome proliferator-activated receptor-γ2 did not offer the same ‘protective’ role in Indians as it did in Caucasians60. Such contradictory associations of an allele provide evidence for heterogeneity in different populations and underscore the need for carrying out association studies in diverse ethnic populations to draw more accurate conclusions in each population.
Mechanistic basis for elevated BP level in the carriers of 364Ser: influence of the endothelial NO pathway
It is well established that the endothelium plays an important role in regulating arterial BP. The manifestation of hypertension through impaired endothelial-dependent vasodilation as well as reduced NO production has been well-documented in both animal 61, 62 and human 63, 64 studies. It is, therefore, not surprising that the hypertensive and hyperadrenergic phenotype in the CHGA-KO mice was accompanied by lowered NO levels65. The attenuation of such a phenotype on the exogenous administration of CST would therefore have to route through restoration of NO levels. In a study carried out in BAE-1 (bovine aortic endothelium) cells20, CST-WT was shown to induce a wortmannin-sensitive, Ca2+-independent increase in NO production and eNOS Ser1179 phosphorylation while CST-364Ser was, found to be ineffective. CST-WT has also been shown to dose-dependently induce a NO-cGMP dependent cardio-suppression in the in vitro frog heart66. In light of this, we asked whether CST-WT and CST-364Ser differ in their ability to generate NO in HUVECs. Indeed, CST-364Ser displayed lower efficacy to produce NO in HUVECs (Fig. 2D), thus corroborating the elevated BP levels in the 364Ser allele carrying individuals. Consistently, carriers of 364Ser allele showed diminished (by ~1090 mm/sec) brachial artery pulse-wave velocity (indicating increased endothelial dysfunction) in a Japanese population59. eNOS is known to be activated via phosphorylation at its Ser1177 residue71, 7267, 68. HUVECs treated with CST-WT showed increased levels of phosphorylation at Ser1177 sites of eNOS as compared to HUVECs treated with CST-364Ser (Fig. 2G). In case of CST-WT, there was a ~3.8-fold increase in Ser1177 phosphorylation levels over basal; CST-364Ser, on the other hand, showed only a ~2.2-fold increase in Ser1177 phosphorylation levels over basal. This is indicative of increased eNOS activity in case of CST-WT as compared to CST-364Ser which goes in corroboration with our observations of increased NO levels on treatment with CST-WT as compared to CST-364Ser in HUVECs.
Direct links between stimulation of cardiovascular beta-adrenergic receptors and NO generation in endothelial cells are well-established41. In isolated human umbilical vein, the vasorelaxation response to either the non-selective beta-adrenergic agonist isoproterenol or to the cAMP analogue dibutyryl cAMP is attenuated by the NOS inhibitor NG-monomethyl-L-arginine (L-NMMA). Thus, the beta-adrenergic-receptor-mediated vasorelaxation response in this system seems to be largely NO-dependent and mediated through the elevation of cAMP levels69. Likewise, in HUVECs as well, it has been shown that either beta-adrenergic-receptor-stimulated or beta-adrenergic-receptor-independent elevation of intracellular cAMP levels results in increased NOS activity69. In HUVECS, the inhibition of eNOS activity in the presence of the ADRB2 antagonist ICI 118551 and not ADRB1 antagonist CGP 20712 shows that this effect is mediated exclusively through ADRB270. Consistently, we found that the elevation in NO levels mediated by CST-WT peptide was abrogated in the presence of ICI 118551 but not CGP 20712 (Fig. 2F and Supp. Fig. S3).
Based on the above findings, we postulated that our in vitro observation of differential effects of CST peptides on NO production via regulation of eNOS activity in HUVECs might be due to the differential yet direct binding of CST peptides to the ADRB2. Indeed, our computational analysis showed that, by virtue of differences in secondary structures, CST-WT blocks the binding of the ligand to ADRB2 (by competing with it for the active site) while CST-364Ser binds at a site that keeps the agonist binding pocket within ADRB2 intact (Fig. 4C). Consistent with the computational prediction, competitive binding assays showed that while CST-WT was able to significantly displace the high-affinity beta-adrenergic receptor ligand cyanopindolol, CST-364Ser failed to do the same even at high concentration (Fig. 3B). Furthermore, the inhibition of agonist isoproterenol-stimulated increase in phospho-ERK levels by CST-WT (but not by CST-364Ser) in ADRB2 HEK-293 cells points towards an anti-adrenergic role of the wild-type peptide but not the variant peptide (Fig. 3C and 3D). This is consistent with a previous report that CST-WT lowers the phospho-ERK levels in Langerdoff-perfused rat hearts21.
In contrast to the effective ability of CST-WT to bind to ADRB2, our computational studies show that CST peptides bind to the outer surface of the ADRB1 receptor, and are thus incapable of blocking the agonist binding pocket in the receptor. These observations are further supported by our competitive binding assays with ADRB1 (Supp. Fig. S4B). Thus, the anti-adrenergic role of CST-WT seems to be mediated primarily through the ADRB2 receptor and may underlie the differential blood pressures observed with the variants being expressed in patients.
CONCLUSIONS
We discovered a naturally-occurring, common genetic variation, Gly364Ser, within the anti-hypertensive peptide catestatin [CST], a proteolytic fragment of the prohormone chromogranin A that is expressed in secretory vesicles of endocrine, neuroendocrine and neuronal cell types. The 364Ser allele showed association with elevated levels of systolic blood pressure, diastolic blood pressure and mean arterial pressure in human subjects across two independent and ethnically/geographically distinct Indian populations. Corroborating these findings, the carriers of the 364Ser allele displayed enhanced risk for hypertension. This is on same lines of a recent Japanese study which found similar associations of the 364Ser allele with hypertension in their population. Genetic association studies of this chromogranin A locus with hypertension and other metabolic diseases need to be carried out in additional ethnic populations to evaluate whether the results are of general importance across the overall world population as well. Our in cella and in silico analyses provided molecular/mechanistic underpinnings for the diminished effects of the CST-364Ser peptide (as compared to the CST-WT peptide) in the modulation of the endothelial NO pathway (via differential binding to ADRB2 and differential activation of ERK and eNOS phosphorylation) that might lead to an increased disease risk in carriers of the 364Ser allele. A schematic of our hypothesis/findings are presented in Fig. 6. These results have implications for inter-individual variations in blood pressure homeostasis and ultimately for pathogenesis of hypertension.
Figure 6. A schematic representation of the plausible mechanistic basis for the effects of CST peptides on BP via modulation of NO pathway.
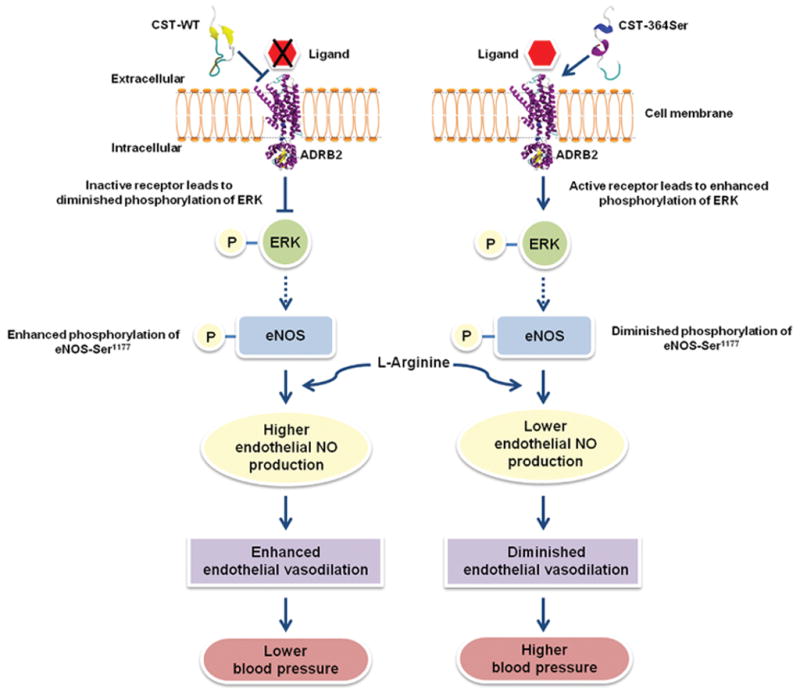
The CST-364Ser peptide does not interact at the ligand binding site of ADRB2 unlike CST-WT owing to differences in their secondary structures. Their differential interactions with ADRB2 result in diminished antagonization of ADRB2 and enhanced activation/ phosphorylation of ERK by CST-364Ser. The altered ERK activation between the CST peptides may result in diminished phosphorylation of eNOS-Ser1177 and consequently lower eNOS activity in the case of CST-364Ser. These cellular/molecular processes lower the NO levels in vascular endothelial cells in the carriers of CST 364Ser allele leading to endothelial dysfunction and thereby increasing their risk for hypertension.
PERSPECTIVES
The neuroendocrine secretory granule protein chromogranin A is emerging as an important regulator of cardiovascular pathophysiology; it acts as precursor for several bioactive peptides including the anti-hypertensive and cardioprotective peptide catestatin. We discovered a non-synonymous genetic variation (Gly364Ser that occurs in a large section of the Worldwide human population) within the catestatin domain. The 364Ser allele was associated with profound elevated blood pressure (up to ~8 mmHg systolic and ~ 6 mmHg diastolic) and enhanced risk (by ~48%) for hypertension in its carriers in several Asian populations. These findings contribute towards potential clinical use of functional genetic variations to predict the risk for hypertension and preventive intervention in asymptomatic patients for better management of cardiovascular disease burden.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What is new?
This is the first study that analyzes the association of the Gly364Ser variant in the anti-hypertensive peptide catestatin with the risk for hypertension in independent Asian populations.
The present study provides evidence for the direct interaction of catestatin peptides with beta-2 adrenergic receptor for the first time to our knowledge.
What is relevant?
This study identifies a novel blood-pressure regulating locus that seems to play an important role to alter the risk for hypertension in several Asian populations.
Summary
Directionally-concordant replication of the association of catestatin 364Ser variant allele with elevated blood pressure in independent human populations suggests a causal role for this genetic variant. Consistently, the 364Ser allele enhanced the risk for hypertension in these study populations. Moreover, our receptor-peptide interaction studies provided evidence for differential interactions of the wild-type and variant catestatin peptides with beta-2 adrenergic receptor that might be responsible for the altered risk for hypertension in their carriers.
Acknowledgments
We acknowledge all the individuals who voluntarily participated in this study. We thank the high performance computational facility at Indian Institute of Technology Madras (IITM). We also thank Suvro Chatterjee, Anna University, Chennai for his help. MK and RK thank Council of Scientific and Industrial Research (CSIR) for fellowship. PKRA and DV received research fellowship from University Grants Commission (UGC). VRC, LS and SS would like to thank IITM, Department of Science and Technology (DST) and Indian Council of Medical Research (ICMR) for fellowship, respectively.
SOURCES OF FUNDING
This work was supported by grants BT/PR9546/MED/12/349/2007 and BT/PR12820/BRB/10/726/2009 from the Department of Biotechnology (DBT), Govt. of India. This work was also supported in part by grants SR/SO/HS-084/2013A from the Science and Engineering Research Board, BT/PR4820/MED/12/622/2013 from DBT, Govt. of India, and NIH RO1 HL HL089473.
NON-STANDARD ABBREVIATIONS AND ACRONYMNS
- CHGA
chromogranin A
- CST
catestatin peptide
- CST-WT
catestatin wild-type peptide
- CST-364Ser
catestatin variant peptide
- ADRB1
beta1-adrenergic receptor
- ADRB2
beta2-adrenergic receptor
- ERK
extracellular regulated kinase
- NO
nitric oxide
- eNOS
endothelial nitric oxide synthase
- MAF
minor allele frequency
- LD
linkage disequilibrium
- DM
type-2 diabetes mellitus
- HTN
essential hypertension
- CAD
coronary artery disease
- HUVECs
human umbilical vein endothelial cells
- HEK
human embryonic kidney cells
- DAF-2 DA
diaminofluorescein diacetate
Footnotes
DISCLOSURE(S)
None
References
- 1.Kim T, Zhang CF, Sun Z, Wu H, Loh YP. Chromogranin a deficiency in transgenic mice leads to aberrant chromaffin granule biogenesis. J Neurosci. 2005;25:6958–6961. doi: 10.1523/JNEUROSCI.1058-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Connor DT, Takiyyuddin MA, Printz MP, Dinh TQ, Barbosa JA, Rozansky DJ, Mahata SK, Wu H, Kennedy BP, Ziegler MG, Wright FA, Schlager G, Parmer RJ. Catecholamine storage vesicle protein expression in genetic hypertension. Blood Press. 1999;8:285–295. doi: 10.1080/080370599439508. [DOI] [PubMed] [Google Scholar]
- 3.Takiyyuddin MA, De Nicola L, Gabbai FB, Dinh TQ, Kennedy B, Ziegler MG, Sabban EL, Parmer RJ, O’Connor DT. Catecholamine secretory vesicles. Augmented chromogranins and amines in secondary hypertension. Hypertension. 1993;21:674–679. doi: 10.1161/01.hyp.21.5.674. [DOI] [PubMed] [Google Scholar]
- 4.Estensen ME, Hognestad A, Syversen U, Squire I, Ng L, Kjekshus J, Dickstein K, Omland T. Prognostic value of plasma chromogranin a levels in patients with complicated myocardial infarction. Am Heart J. 2006;152:e1–6. doi: 10.1016/j.ahj.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 5.Jansson AM, Rosjo H, Omland T, Karlsson T, Hartford M, Flyvbjerg A, Caidahl K. Prognostic value of circulating chromogranin a levels in acute coronary syndromes. Eur Heart J. 2009;30:25–32. doi: 10.1093/eurheartj/ehn513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ceconi C, Ferrari R, Bachetti T, Opasich C, Volterrani M, Colombo B, Parrinello G, Corti A. Chromogranin a in heart failure; a novel neurohumoral factor and a predictor for mortality. Eur Heart J. 2002;23:967–974. doi: 10.1053/euhj.2001.2977. [DOI] [PubMed] [Google Scholar]
- 7.Barbosa JA, Gill BM, Takiyyuddin MA, O’Connor DT. Chromogranin a: Posttranslational modifications in secretory granules. Endocrinology. 1991;128:174–190. doi: 10.1210/endo-128-1-174. [DOI] [PubMed] [Google Scholar]
- 8.Aardal S, Helle KB, Elsayed S, Reed RK, Serck-Hanssen G. Vasostatins, comprising the n-terminal domain of chromogranin a, suppress tension in isolated human blood vessel segments. J Neuroendocrinol. 1993;5:405–412. doi: 10.1111/j.1365-2826.1993.tb00501.x. [DOI] [PubMed] [Google Scholar]
- 9.Tatemoto K, Efendic S, Mutt V, Makk G, Feistner GJ, Barchas JD. Pancreastatin, a novel pancreatic peptide that inhibits insulin secretion. Nature. 1986;324:476–478. doi: 10.1038/324476a0. [DOI] [PubMed] [Google Scholar]
- 10.Fasciotto BH, Trauss CA, Greeley GH, Cohn DV. Parastatin (porcine chromogranin a347–419), a novel chromogranin a-derived peptide, inhibits parathyroid cell secretion. Endocrinology. 1993;133:461–466. doi: 10.1210/endo.133.2.8344192. [DOI] [PubMed] [Google Scholar]
- 11.Loh YP, Koshimizu H, Cawley NX, Tota B. Serpinins: Role in granule biogenesis, inhibition of cell death and cardiac function. Curr Med Chem. 2012;19:4086–4092. doi: 10.2174/092986712802429957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simon JP, Bader MF, Aunis D. Secretion from chromaffin cells is controlled by chromogranin a-derived peptides. Proc Natl Acad Sci U S A. 1988;85:1712–1716. doi: 10.1073/pnas.85.5.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahata SK, O’Connor DT, Mahata M, Yoo SH, Taupenot L, Wu H, Gill BM, Parmer RJ. Novel autocrine feedback control of catecholamine release. A discrete chromogranin a fragment is a noncompetitive nicotinic cholinergic antagonist. J Clin Invest. 1997;100:1623–1633. doi: 10.1172/JCI119686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahata SK, Mahata M, Wen G, Wong WB, Mahapatra NR, Hamilton BA, O’Connor DT. The catecholamine release-inhibitory “catestatin” fragment of chromogranin a: Naturally occurring human variants with different potencies for multiple chromaffin cell nicotinic cholinergic responses. Mol Pharmacol. 2004;66:1180–1191. doi: 10.1124/mol.104.002139. [DOI] [PubMed] [Google Scholar]
- 15.Sahu BS, Mohan J, Sahu G, Singh PK, Sonawane PJ, Sasi BK, Allu PK, Maji SK, Bera AK, Senapati S, Mahapatra NR. Molecular interactions of the physiological anti-hypertensive peptide catestatin with the neuronal nicotinic acetylcholine receptor. J Cell Sci. 2012;125:2323–2337. doi: 10.1242/jcs.103176. [DOI] [PubMed] [Google Scholar]
- 16.O’Connor DT, Kailasam MT, Kennedy BP, Ziegler MG, Yanaihara N, Parmer RJ. Early decline in the catecholamine release-inhibitory peptide catestatin in humans at genetic risk of hypertension. J Hypertens. 2002;20:1335–1345. doi: 10.1097/00004872-200207000-00020. [DOI] [PubMed] [Google Scholar]
- 17.Mahapatra NR, O’Connor DT, Vaingankar SM, Hikim AP, Mahata M, Ray S, Staite E, Wu H, Gu Y, Dalton N, Kennedy BP, Ziegler MG, Ross J, Mahata SK. Hypertension from targeted ablation of chromogranin a can be rescued by the human ortholog. J Clin Invest. 2005;115:1942–1952. doi: 10.1172/JCI24354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wen G, Mahata SK, Cadman P, Mahata M, Ghosh S, Mahapatra NR, Rao F, Stridsberg M, Smith DW, Mahboubi P, Schork NJ, O’Connor DT, Hamilton BA. Both rare and common polymorphisms contribute functional variation at chga, a regulator of catecholamine physiology. Am J Hum Genet. 2004;74:197–207. doi: 10.1086/381399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sahu BS, Obbineni JM, Sahu G, Allu PK, Subramanian L, Sonawane PJ, Singh PK, Sasi BK, Senapati S, Maji SK, Bera AK, Gomathi BS, Mullasari AS, Mahapatra NR. Functional genetic variants of the catecholamine-release-inhibitory peptide catestatin in an indian population: Allele-specific effects on metabolic traits. J Biol Chem. 2012;287:43840–43852. doi: 10.1074/jbc.M112.407916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bassino E, Fornero S, Gallo MP, Ramella R, Mahata SK, Tota B, Levi R, Alloatti G. A novel catestatin-induced antiadrenergic mechanism triggered by the endothelial pi3k-enos pathway in the myocardium. Cardiovasc Res. 2011;91:617–624. doi: 10.1093/cvr/cvr129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Angelone T, Quintieri AM, Brar BK, Limchaiyawat PT, Tota B, Mahata SK, Cerra MC. The antihypertensive chromogranin a peptide catestatin acts as a novel endocrine/paracrine modulator of cardiac inotropism and lusitropism. Endocrinology. 2008;149:4780–4793. doi: 10.1210/en.2008-0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mazza R, Gattuso A, Mannarino C, Brar BK, Barbieri SF, Tota B, Mahata SK. Catestatin (chromogranin a344–364) is a novel cardiosuppressive agent: Inhibition of isoproterenol and endothelin signaling in the frog heart. Am J Physiol Heart Circ Physiol. 2008;295:H113–H122. doi: 10.1152/ajpheart.00172.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Imbrogno S, Garofalo F, Cerra MC, Mahata SK, Tota B. The catecholamine release-inhibitory peptide catestatin (chromogranin a344–363) modulates myocardial function in fish. J Exp Biol. 2010;213:3636–3643. doi: 10.1242/jeb.045567. [DOI] [PubMed] [Google Scholar]
- 24.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: Analysis and visualization of ld and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 25.Gauderman WJ. Sample size requirements for matched case-control studies of gene-environment interaction. Stat Med. 2002;21:35–50. doi: 10.1002/sim.973. [DOI] [PubMed] [Google Scholar]
- 26.Giri H, Muthuramu I, Dhar M, Rathnakumar K, Ram U, Dixit M. Protein tyrosine phosphatase shp2 mediates chronic insulin-induced endothelial inflammation. Arterioscler Thromb Vasc Biol. 2012;32:1943–1950. doi: 10.1161/ATVBAHA.111.239251. [DOI] [PubMed] [Google Scholar]
- 27.Kesavan R, Potunuru UR, Nastasijevic B, TA, Joksic G, Dixit M. Inhibition of vascular smooth muscle cell proliferation by gentiana lutea root extracts. PLoS One. 2013;8:e61393. doi: 10.1371/journal.pone.0061393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Noma T, Lemaire A, Naga Prasad SV, Barki-Harrington L, Tilley DG, Chen J, Le Corvoisier P, Violin JD, Wei H, Lefkowitz RJ, Rockman HA. Beta-arrestin-mediated beta1-adrenergic receptor transactivation of the egfr confers cardioprotection. J Clin Invest. 2007;117:2445–2458. doi: 10.1172/JCI31901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naga Prasad SV, Barak LS, Rapacciuolo A, Caron MG, Rockman HA. Agonist-dependent recruitment of phosphoinositide 3-kinase to the membrane by beta-adrenergic receptor kinase 1. A role in receptor sequestration. J Biol Chem. 2001;276:18953–18959. doi: 10.1074/jbc.M102376200. [DOI] [PubMed] [Google Scholar]
- 30.Vasudevan NT, Mohan ML, Gupta MK, Hussain AK, Naga Prasad SV. Inhibition of protein phosphatase 2a activity by pi3kgamma regulates beta-adrenergic receptor function. Mol Cell. 2011;41:636–648. doi: 10.1016/j.molcel.2011.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cherezov V, Rosenbaum DM, Hanson MA, Rasmussen SG, Thian FS, Kobilka TS, Choi HJ, Kuhn P, Weis WI, Kobilka BK, Stevens RC. High-resolution crystal structure of an engineered human beta2-adrenergic g protein-coupled receptor. Science. 2007;318:1258–1265. doi: 10.1126/science.1150577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Allu PK, Chirasani VR, Ghosh D, Mani A, Bera AK, Maji SK, Senapati S, Mullasari AS, Mahapatra NR. Naturally occurring variants of the dysglycemic peptide pancreastatin: Differential potencies for multiple cellular functions and structure-function correlation. J Biol Chem. 2014;289:4455–4469. doi: 10.1074/jbc.M113.520916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Webb B, Sali A. Comparative protein structure modeling using modeller. Curr Protoc Bioinformatics. 2014;47:1–5. doi: 10.1002/0471250953.bi0506s47. [DOI] [PubMed] [Google Scholar]
- 34.Pierce BG, Wiehe K, Hwang H, Kim BH, Vreven T, Weng Z. Zdock server: Interactive docking prediction of protein-protein complexes and symmetric multimers. Bioinformatics. 2014;30:1771–1773. doi: 10.1093/bioinformatics/btu097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Humphrey W, Dalke A, Schulten K. Vmd: Visual molecular dynamics. J Mol Graph. 1996;14:33–38. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- 36.de Beer TA, Berka K, Thornton JM, Laskowski RA. Pdbsum additions. Nucleic Acids Res. 2014;42:D292–D296. doi: 10.1093/nar/gkt940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laskowski RA, Swindells MB. Ligplot+: Multiple ligand-protein interaction diagrams for drug discovery. J Chem Inf Model. 2011;51:2778–2786. doi: 10.1021/ci200227u. [DOI] [PubMed] [Google Scholar]
- 38.Cui JS, Hopper JL, Harrap SB. Antihypertensive treatments obscure familial contributions to blood pressure variation. Hypertension. 2003;41:207–210. doi: 10.1161/01.hyp.0000044938.94050.e3. [DOI] [PubMed] [Google Scholar]
- 39.Rao F, Wen G, Gayen JR, et al. Catecholamine release-inhibitory peptide catestatin (chromogranin a(352–372)): Naturally occurring amino acid variant gly364ser causes profound changes in human autonomic activity and alters risk for hypertension. Circulation. 2007;115:2271–2281. doi: 10.1161/CIRCULATIONAHA.106.628859. [DOI] [PubMed] [Google Scholar]
- 40.Taupenot L, Harper KL, O’Connor DT. The chromogranin-secretogranin family. N Engl J Med. 2003;348:1134–1149. doi: 10.1056/NEJMra021405. [DOI] [PubMed] [Google Scholar]
- 41.Queen LR, Ferro A. Beta-adrenergic receptors and nitric oxide generation in the cardiovascular system. Cell Mol Life Sci. 2006;63:1070–1083. doi: 10.1007/s00018-005-5451-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ. Autodock4 and autodocktools4: Automated docking with selective receptor flexibility. J Comput Chem. 2009;30:2785–2791. doi: 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller-Gallacher JL, Nehme R, Warne T, Edwards PC, Schertler GF, Leslie AG, Tate CG. The 2.1 a resolution structure of cyanopindolol-bound beta1-adrenoceptor identifies an intramembrane na+ ion that stabilises the ligand-free receptor. PLoS One. 2014;9:e92727. doi: 10.1371/journal.pone.0092727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, Kondrashov AS, Sunyaev SR. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7:248–249. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mahapatra NR. Catestatin is a novel endogenous peptide that regulates cardiac function and blood pressure. Cardiovasc Res. 2008;80:330–338. doi: 10.1093/cvr/cvn155. [DOI] [PubMed] [Google Scholar]
- 46.Mazza R, Tota B, Gattuso A. Cardio-vascular activity of catestatin: Interlocking the puzzle pieces. Curr Med Chem. 2015;22:292–304. doi: 10.2174/0929867321666141106114928. [DOI] [PubMed] [Google Scholar]
- 47.Sahu BS, Sonawane PJ, Mahapatra NR. Chromogranin a: A novel susceptibility gene for essential hypertension. Cell Mol Life Sci. 2010;67:861–874. doi: 10.1007/s00018-009-0208-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gayen JR, Gu Y, O’Connor DT, Mahata SK. Global disturbances in autonomic function yield cardiovascular instability and hypertension in the chromogranin a null mouse. Endocrinology. 2009;150:5027–5035. doi: 10.1210/en.2009-0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kennedy BP, Mahata SK, O’Connor DT, Ziegler MG. Mechanism of cardiovascular actions of the chromogranin a fragment catestatin in vivo. Peptides. 1998;19:1241–1248. doi: 10.1016/s0196-9781(98)00086-2. [DOI] [PubMed] [Google Scholar]
- 50.Fung MM, Salem RM, Mehtani P, Thomas B, Lu CF, Perez B, Rao F, Stridsberg M, Ziegler MG, Mahata SK, O’Connor DT. Direct vasoactive effects of the chromogranin a (chga) peptide catestatin in humans in vivo. Clin Exp Hypertens. 2010;32:278–287. doi: 10.3109/10641960903265246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gaede AH, Pilowsky PM. Catestatin in rat rvlm is sympathoexcitatory, increases barosensitivity, and attenuates chemosensitivity and the somatosympathetic reflex. Am J Physiol Regul Integr Comp Physiol. 2010;299:R1538–1545. doi: 10.1152/ajpregu.00335.2010. [DOI] [PubMed] [Google Scholar]
- 52.Gaede AH, Pilowsky PM. Catestatin, a chromogranin a-derived peptide, is sympathoinhibitory and attenuates sympathetic barosensitivity and the chemoreflex in rat cvlm. Am J Physiol Regul Integr Comp Physiol. 2012;302:R365–372. doi: 10.1152/ajpregu.00409.2011. [DOI] [PubMed] [Google Scholar]
- 53.Dorajoo R, Blakemore AI, Sim X, Ong RT, Ng DP, Seielstad M, Wong TY, Saw SM, Froguel P, Liu J, Tai ES. Replication of 13 obesity loci among singaporean chinese, malay and asian-indian populations. Int J Obes. 2012;36:159–163. doi: 10.1038/ijo.2011.86. [DOI] [PubMed] [Google Scholar]
- 54.Ntzani EE, Liberopoulos G, Manolio TA, Ioannidis JP. Consistency of genome-wide associations across major ancestral groups. Hum Genet. 2012;131:1057–1071. doi: 10.1007/s00439-011-1124-4. [DOI] [PubMed] [Google Scholar]
- 55.Carlson CS, Matise TC, North KE, et al. Generalization and dilution of association results from european gwas in populations of non-european ancestry: The page study. PLoS Biol. 2013;11:e1001661. doi: 10.1371/journal.pbio.1001661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hiura Y, Tabara Y, Kokubo Y, Okamura T, Miki T, Tomoike H, Iwai N. A genome-wide association study of hypertension-related phenotypes in a japanese population. Circ J. 2010;74:2353–2359. doi: 10.1253/circj.cj-10-0353. [DOI] [PubMed] [Google Scholar]
- 57.Guo Y, Tomlinson B, Chu T, Fang YJ, Gui H, Tang CS, Yip BH, Cherny SS, Hur YM, Sham PC, Lam TH, Thomas NG. A genome-wide linkage and association scan reveals novel loci for hypertension and blood pressure traits. PLoS One. 2012;7:e31489. doi: 10.1371/journal.pone.0031489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Takiyyuddin MA, Parmer RJ, Kailasam MT, et al. Chromogranin a in human hypertension. Influence of heredity. Hypertension. 1995;26:213–220. doi: 10.1161/01.hyp.26.1.213. [DOI] [PubMed] [Google Scholar]
- 59.Choi Y, Miura M, Nakata Y, et al. A common genetic variant of the chromogranin a-derived peptide catestatin is associated with atherogenesis and hypertension in a japanese population. Endocr J. 2015;62:797–804. doi: 10.1507/endocrj.EJ14-0471. [DOI] [PubMed] [Google Scholar]
- 60.Radha V, Vimaleswaran KS, Babu HN, Abate N, Chandalia M, Satija P, Grundy SM, Ghosh S, Majumder PP, Deepa R, Rao SM, Mohan V. Role of genetic polymorphism peroxisome proliferator-activated receptor-gamma2 pro12ala on ethnic susceptibility to diabetes in south-asian and caucasian subjects: Evidence for heterogeneity. Diabetes Care. 2006;29:1046–1051. doi: 10.2337/diacare.2951046. [DOI] [PubMed] [Google Scholar]
- 61.Luscher TF, Diederich D, Weber E, Vanhoutte PM, Buhler FR. Endothelium-dependent responses in carotid and renal arteries of normotensive and hypertensive rats. Hypertension. 1988;11:573–578. doi: 10.1161/01.hyp.11.6.573. [DOI] [PubMed] [Google Scholar]
- 62.Fu-Xiang D, Jameson M, Skopec J, Diederich A, Diederich D. Endothelial dysfunction of resistance arteries of spontaneously hypertensive rats. J Cardiovasc Pharmacol. 1992;20:S190–192. doi: 10.1097/00005344-199204002-00053. [DOI] [PubMed] [Google Scholar]
- 63.Panza JA, Casino PR, Kilcoyne CM, Quyyumi AA. Role of endothelium-derived nitric oxide in the abnormal endothelium-dependent vascular relaxation of patients with essential hypertension. Circulation. 1993;87:1468–1474. doi: 10.1161/01.cir.87.5.1468. [DOI] [PubMed] [Google Scholar]
- 64.Panza JA, Garcia CE, Kilcoyne CM, Quyyumi AA, Cannon RO., 3rd Impaired endothelium-dependent vasodilation in patients with essential hypertension. Evidence that nitric oxide abnormality is not localized to a single signal transduction pathway. Circulation. 1995;91:1732–1738. doi: 10.1161/01.cir.91.6.1732. [DOI] [PubMed] [Google Scholar]
- 65.Gayen JR, Zhang K, RamachandraRao SP, Mahata M, Chen Y, Kim HS, Naviaux RK, Sharma K, Mahata SK, O’Connor DT. Role of reactive oxygen species in hyperadrenergic hypertension: Biochemical, physiological, and pharmacological evidence from targeted ablation of the chromogranin a (chga) gene. Circ Cardiovasc Genet. 2010;3:414–425. doi: 10.1161/CIRCGENETICS.109.924050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mazza R, Pasqua T, Gattuso A. Cardiac heterometric response: The interplay between catestatin and nitric oxide deciphered by the frog heart. Nitric Oxide. 2012;27:40–49. doi: 10.1016/j.niox.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 67.Michell BJ, Chen Z, Tiganis T, Stapleton D, Katsis F, Power DA, Sim AT, Kemp BE. Coordinated control of endothelial nitric-oxide synthase phosphorylation by protein kinase c and the camp-dependent protein kinase. J Biol Chem. 2001;276:17625–17628. doi: 10.1074/jbc.C100122200. [DOI] [PubMed] [Google Scholar]
- 68.Butt E, Bernhardt M, Smolenski A, Kotsonis P, Frohlich LG, Sickmann A, Meyer HE, Lohmann SM, Schmidt HH. Endothelial nitric-oxide synthase (type iii) is activated and becomes calcium independent upon phosphorylation by cyclic nucleotide-dependent protein kinases. J Biol Chem. 2000;275:5179–5187. doi: 10.1074/jbc.275.7.5179. [DOI] [PubMed] [Google Scholar]
- 69.Ferro A, Queen LR, Priest RM, Xu B, Ritter JM, Poston L, Ward JP. Activation of nitric oxide synthase by beta 2-adrenoceptors in human umbilical vein endothelium in vitro. Br J Pharmacol. 1999;126:1872–1880. doi: 10.1038/sj.bjp.0702512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yao K, Xu B, Liu YP, Ferro A. Effects of beta-adrenoceptor stimulation on endothelial nitric-oxide synthase phosphorylation of human umbilical vein endothelial cells. Acta Pharmacol Sin. 2003;24:219–224. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.