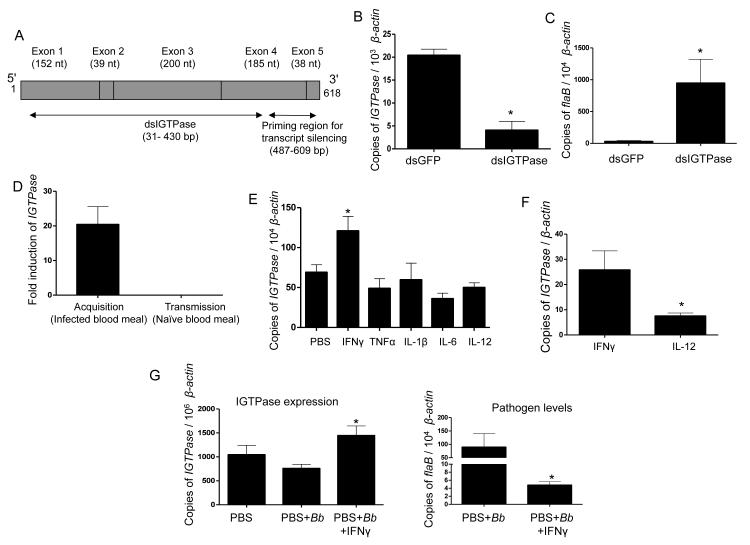
Figure 2. IGTPase influences persistence of B. burgdorferi in feeding ticks and is induced by a mammalian cytokine.
(A) Schematic representation of IGTPase open reading frame showing multiple exons and regions targeted for RNA interference. Exons (gray boxes), regions for dsRNA, and primers for assessment of transcript silencing are shown.
(B) Knockdown of IGTPase transcripts via RNA interference. Nymphal ticks (25/group) were injected with IGTPase dsRNA or control GFP dsRNA, allowed to feed on infected mice and collected after feeding. IGTPase transcript levels in gut tissues were measured by quantitative RT-PCR and normalized for tick β-actin. The data represent the mean ± SEM values from five individual ticks. * p < 0.0001.
(C) Silencing of IGTPase expression results in higher levels of B. burgdorferi in ticks. Nymphs were microinjected with IGTPase dsRNA or GFP dsRNA, allowed to parasitize B. burgdorferi-infected mice and spirochete burden in ticks was detected by measuring flaB transcripts normalized with tick β-actin. The mean ± SEM values from five individual ticks are presented. * p < 0.05.
(D) Selective induction of IGTPase during B. burgdorferi acquisition from infected mice. Naïve nymphal ticks that engorged on B. burgdorferi-infected mice for 48 hours show an average of 20-fold higher IGTPase transcript levels compared to naïve ticks or to B. burgdorferi-infected ticks that parasitized uninfected hosts during spirochete transmission. The data represent the mean ± SEM values from pools of 20 ticks per group.
(E) Expression of IGTPase is induced by a mammalian cytokine. Groups of nymphal ticks were microinjected with PBS (control) or a panel of mammalian cytokines induced during B. burgdorferi infection and allowed to parasitize naïve mice for 48 hours. The amounts of IGTPase transcripts normalized against tick β-actin were then analyzed by qRT-PCR. The data presented correspond to at least three ticks from each group. * p < 0.02.
(F) Expression of IGTPase is significantly induced in the presence of IFNγ. Groups of nymphal ticks (25/group) were injected with IFNγ or a control cytokine (IL-12) and examined for upregulation of IGTPase transcripts, as described for Figure 2E. The data represent the mean ± SEM values from 10 individual ticks. * p < 0.05.
(G) IGTPase induction and its spirochete control require the presence of IFNγ. Groups of nymphal ticks (25/group) were injected with buffer (PBS), B. burgdorferi, or B. burgdorferi plus IFNγ, and examined for upregulation of IGTPase transcripts (left panels) and spirochete levels (right panels), as described for Figure 2E and 2C, respectively. The data represent the mean ± SEM values from 10 individual ticks. * p < 0.05.