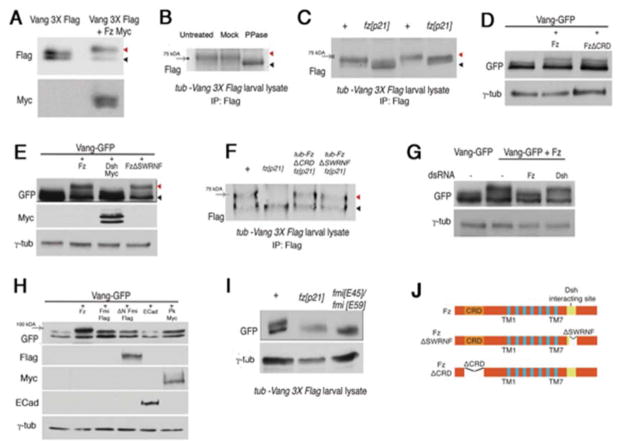
Figure 1. Fz specifically promotes phosphorylation of Vang.
(A–C) Vang is phosphorylated in a Fz-dependent manner. (A) Western blot analysis of Vang3XFlag and Vang3XFlag co-transfected with Fz-myc in S2 cells. Co-transfection of Fz causes a shift in the upper band of the Vang3XFlag doublet (red arrowhead; black arrowhead marks unshifted band). (B) Treatment of larval lysate with lambda protein phosphatase eliminates the Vang band shift (black arrowhead), indicating that it is caused by phosphorylation. The 75kDa molecular weight marker is indicated by the gray arrow. (C) Western blot of in vivo lysate from tub-Vang3XFlag larvae in a wild-type and homozygous fz null mutant background (fzP21). In the absence of Fz, the Vang band shift is not observed (shifted bands are marked with red arrowhead, unshifted bands marked with black arrowhead). See Fig. S1 for examples of full blots.
(D) Cell autonomous Fz-requirement for Vang phosphorylation: FzΔCRD induces a VangGFP band shift, similar to wild-type Fz in S2 cells. (E) Cotransfection of Dsh with Vang in S2 cells does not induce a Vang-GFP band shift. FzΔSWRNF (lacking Dsh interacting site) induces a band shift, indistinguishable from wt Fz.
(F) Fz dependent Vang phosphorylation is cell-autonomous and Dsh-independent in vivo. Larval lysate from fzp21 mutant flies expressing tub-FzΔCRD or tub-FzΔSWRNF display a band-shift similar to wild-type. (G) Knockdown of Dsh via dsRNA in S2 cells does not affect the Fz induced Vang-GFP band shift.
(H) Cotransfection of either Fmi, ΔN-Fmi, E-Cad, or Pk with Vang in S2 cells does not induce a Vang-GFP band shift.
(I) In vivo lysates from tub-VangGFP expressing wing discs do not show a Vang band shift in a fmi mutant background (fmiE45; GAL4-1407/fmiE59; UAS-Fmi: Fmi was expressed from the GAL4-1407 driver to rescue embryonic and larval lethality).
(J) Schematic presentation of wild-type Fz and Fz mutant isoforms used in the above experiments. Trans-membrane domains are shown in blue. FzΔSWRNF lacks the Dsh interacting site, and FzΔCRD lacks the CRD (N-terminal cysteine-rich domain) required for non-autonomous Fz signaling and function.