Abstract
Skilled reading depends on recognizing words efficiently in isolation (word-level processing; WL) and extracting meaning from text (discourse-level processing; DL); deficiencies in either result in poor reading. FMRI has revealed consistent overlapping networks in word and passage reading, as well as unique regions for DL processing, however less is known about how WL and DL processes interact. Here we examined functional connectivity from seed regions derived from where BOLD signal overlapped during word and passage reading in 38 adolescents ranging in reading ability, hypothesizing that even though certain regions support word- and higher-level language, connectivity patterns from overlapping regions would be task modulated. Results indeed revealed that the left-lateralized semantic and working memory (WM) seed regions showed task-dependent functional connectivity patterns: during DL processes, semantic and WM nodes all correlated with the left angular gyrus, a region implicated in semantic memory/coherence building. In contrast, during WL, these nodes coordinated with a traditional WL area (left occipitotemporal region). Additionally, these WL and DL findings were modulated by decoding and comprehension abilities, respectively, with poorer abilities correlating with decreased connectivity. Findings indicate that key regions may uniquely contribute to multiple levels of reading; we speculate that these connectivity patterns may be especially salient for reading outcomes and intervention response.
Introduction
Skilled reading comprehension (RC) requires the integration of word-level (WL) and discourse-level (DL) processing of a text. Early adolescence (~10–14 years old) is a period of reading development marked by a transition in the classroom from a focus on WL reading (“learning to read”) to cohesive integration of WL and DL processes (“reading to learn”). Readers with RC deficits have been found to show correlated but separable difficulties in both word and text reading processes, including vocabulary skills (Cain & Oakhill, 2006; Kate Nation, Snowling, & Clarke, 2007; Spencer, Quinn, & Wagner, 2014), integration of semantic information within and across sentences (Oakhill et al., 2003; Oakhill & Cain, 2012), and working memory and other executive functions (WM; Locascio, Mahone, Eason, & Cutting, 2010.; Nation, Adams, Bowyer-Crane, & Snowling, 1999; Stothard, 1992). These findings suggest that RC deficits may involve unique and interactive deficits in WL and DL processes, particularly semantic processing and WM. Nevertheless, despite the estimated prevalence rate of approximately 30% of adolescent readers struggling with RC (U.S. Department of Education: National Assessment of Educational Progress, 2013), the neurobiological underpinnings of WL and DL functions, and how they are appropriately integrated during reading, is poorly understood. Surprisingly, no neuroimaging studies to date have examined the interaction between WL and DL processes in adolescents. The current study aimed to address this significant gap in the literature by using a naturalistic reading paradigm in order to investigate the relationship between these tiers of reading in adolescent readers who ranged in reading ability.
Surveying the literature for individual WL and DL studies reveals consistent neural networks identified across both levels of reading. The literature has clearly established that WL reading recruits a widespread network of left-lateralized language regions (Price, 2012). These include activation of the left occipitotemporal area (OT; for full abbreviations list see Table 1), particularly the putative visual word form area (pVWFA), which is thought to support orthographic processing, including rapid visual word recognition (McCandliss, Cohen, & Dehaene, 2003; Schlaggar & McCandliss, 2007). Additionally, WL processing recruits phonological support regions, including subregions of the inferior frontal gyrus (IFG; BA 44 in particular), with additional associations with the supramarginal gyrus (SMG; Richlan, 2012;C. J. Price, 2012; Vandermosten, Boets, Wouters, & Ghesquière, 2012). Finally, the middle temporal gyrus (MTG) and temporal pole (TP), and other subcomponents of the IFG (BA 45 and 47 especially) are thought to support WL semantic functions (i.e. the connection of word stimuli to meaning; Price, 2012). In the context of these findings, WL neuroimaging work appears to map reasonably well onto behavioral models of reading, which suggest that skilled reading requires adequate formation and integration of the orthographic, phonological, and semantic representations of words (Perfetti, 2007). In addition to these language-specific functions, behavioral and neuroimaging studies have also suggested that the integration of word representations is supported by top-down or executive processes such as WM (Christopher et al., 2012)—cognitive abilities which are largely associated with the dorsolateral prefrontal cortex (dlPFC; Coelho, Lê, Mozeiko, Krueger, & Grafman, 2012; Fedorenko, 2014). Consequently, the dlPFC could also play an important role in WL processes (Kovelman et al., 2012).
Table 1.
Abbreviations of neural regions
| Abbreviation | Region |
|---|---|
| AG | angular gyrus |
| ACC | anterior cingulate cortex |
| dlPFC | dorsolateral prefrontal cortex |
| dmPFC | dorsomedial prefrontal cortex |
| IFG | inferior frontal gyrus |
| IPL | inferior parietal lobule |
| IPS | intraparietal sulcus |
| ITG | inferior temporal gyrus |
| MFG | middle frontal gyrus |
| MTG | middle temporal gyrus |
| OT | occipitotemporal area |
| PCC | posterior cingulate cortex |
| PCU | precuneus |
| pVWFA | putative visual word form area |
| SFG | superior frontal gyrus |
| SMA | supplementary motor area |
| SMG | supramarginal gyrus |
| SPL | superior parietal lobule |
| STS | superior temporal sulcus |
| TP | temporal pole |
Previous literature has revealed that DL processing involves a complex integration of multiple skill sets spanning different cognitive domains, including those required for WL reading. In addition to the WL processing requirements mentioned above (Kendeou, van den Broek, Helder, & Karlsson, 2014; Perfetti, 2007), in order to comprehend a text meaning must be integrated across multi-word units through combinatorial semantic and syntactic unification (i.e. DL processes; Friederici, 2011; Hagoort & Indefrey, 2014; Humphries, Binder, Medler, & Liebenthal, 2007). This act of building meaning is supported by domain-general executive functions, including WM, inferencing, planning/organization, and social cognition, which work to appropriately maintain, organize, and contextualize the incoming information (Cutting, Materek, Cole, Levine, & Mahone, 2009; Ferstl, Neumann, Bogler, & von Cramon, 2008; Kendeou et al., 2014; Sesma, Mahone, Levine, Eason, & Cutting, 2009). Through these convergent functions, meaning is integrated into an evolving internal representation of the text known as the “situation model” (Dijk & Kintsch, 1983; Whitney et al., 2009). Because reading connected texts requires integration of WL processes, not surprisingly, neuroimaging studies find large areas of overlap between word and passage reading, including left IFG, TP, and MTG (Friederici, 2011; Hagoort & Indefrey, 2014; Price, 2012); the dlPFC has also been implicated (Christopher et al., 2012; Coelho, Lê, Mozeiko, Krueger, & Grafman, 2012; Fedorenko, 2014). Discourse-specific areas include domain-general nodes within the default mode network (DMN) including the dorsal medial prefrontal cortex (dmPFC), bilateral angular gyri (AG), posterior midline regions, and in some cases, the hippocampus and bilateral anterior superior temporal sulcus (Buckner, Andrews-Hanna, & Schacter, 2008). This dispersed network is implicated in numerous cognitive tasks, but in the context of discourse processing, appears to support inferential and contextualization functions, including social cognition (Ferstl et al., 2008; Mar, 2011).
Central to the current study, regions of overlap between WL and DL processing are implicitly interpreted in discourse processing studies as primarily supporting “lower-level” WL processes, (i.e. they are subtracted out using WL baseline tasks). However, studies examining these left-lateralized language and WM areas suggest that these regions are potentially multi-functional, with flexible network properties depending on task demand, or, perhaps due to smaller functionally specific subregions. Indeed, subregions of the IFG form a complex functional gradient potentially supporting a broader role of the IFG in the unification of information (Hagoort, 2005): BA 44 and BA 45 are both implicated in syntactic unification; BA 44 additionally is related to phonological functions; and BA 45 (along with BA 47) is also thought to support semantic processes (Cappa, 2012; Hagoort & Indefrey, 2014; Hagoort, 2005; Price, 2012; Pugh et al., 2001). More generally, IFG, MTG, and TP have been associated with multiple functions including WL reading (Cappa, 2012; Jefferies, 2013; Pugh et al., 2001; Tsapkini et al., 2011), combinatorial semantics (Hagoort & Indefrey, 2014; Humphries et al., 2007), syntax (IFG, MTG, TP; Cappa, 2012; Hagoort & Indefrey, 2014; Hagoort, 2005), semantic storage (MTG; Jefferies, 2013; Price, 2012), and executive semantic functions (Whitney, Kirk, O’Sullivan, Lambon Ralph, & Jefferies, 2011).
Whether these regions are truly multifunctional, or reflect smaller, functionally-specific subregions is an open question. For example, the canonical Broca’s area has been divided into multiple subdivisions based on connectivity, cytoarchitecture, and transmitter receptor distribution (Amunts & Zilles, 2012), some of which map onto proposed, distinct functions (Friederici, 2011). Consequently, areas which appear to exhibit flexible network properties could be comprised of different neuronal subpopulations. To determine whether hub regions exhibit flexible connections due to true multifunctionality or proximal, heterogeneous subdivisions, the dynamic network and functional characteristics of these regions requires close interrogation in the context of reading and reading deficits. From this context, heretofore when we refer to the term multifunctionality, we acknowledge that it may reflect either “true” multifunctionality or further subregions that are proximal but perhaps heterogeneous in function.
In addition to language regions common to WL and DL processing, neuroimaging and behavioral studies suggest that the dlPFC-based top-down, executive control and WM functions may play critical, independent and integrative roles in WL and DL cognition (Christopher et al., 2012; Coelho et al., 2012; Fedorenko, 2014; Locascio, Mahone, Eason, & Cutting, 2010; Newman, Malaia, Seo, & Cheng, 2013; Petten, Weckerly, Mclsaac, & Kutas, 1997). A traditional cognitive model of reading, the simple view of reading (Hoover & Gough, 1990), hypothesizes that skilled RC ability requires appropriate integration of WL and oral DL (listening or language comprehension) processes. An expanded version of this model suggests that this integration is potentially facilitated by executive functions (Cutting et al., 2015). Practically, this integration seems to involve semantic processing, particularly connecting orthographic representations to meaning, and integrating this meaning across units of text. Behavioral studies of RC deficits have suggested that struggling readers could have deficits in this integration process, rather than the component skills of word reading and oral language (Locascio, Mahone, Eason, & Cutting, 2010.; Sesma, Mahone, Levine, Eason, & Cutting, 2009). In this context, the dlPFC may play a role in top-down maintenance of WL and DL integration, specifically by managing semantic-orthographic representations and combinatorial semantic processing.
Thus, previous literature on word and discourse processes indicates that semantic (including orthographic-semantic networks) and executive overlap regions described above may be multi-functional in the context of reading at multiple levels. Due to the dynamic activity of these areas, some studies have suggested that these overlapping language and executive regions are cognitive “hubs” (TP, Patterson, Nestor, & Rogers, 2007; IFG, Bitan et al., 2005; Hagoort & Indefrey, 2014; MTG, Visser, Jefferies, Embleton, & Lambon Ralph, 2012); i.e. they exhibit flexible network activity by communicating with a greater number of disparate networks ,and “support and/or integrate multiple types of information” (Power, Schlaggar, Lessov-Schlaggar, & Petersen, 2013). Importantly, a recent study by Power et al. (2013) suggests that damage to hub regions has a significant impact on clinical outcomes across different cognitive domains. Consequently, examination of multifunctional regions has potentially significant implications both within and beyond the context of reading (Cole et al., 2013). By examining network properties of multifunctional passage and word overlap regions, we expected not only to identify patterns predictive of reading ability, but also sought to better identify potentially flexible network characteristics of hub areas within the traditionally identified language network.
Current Study
In the current study, we used an expository text reading paradigm to examine reading networks in adolescents with a range of word reading and RC abilities. In addition to the inclusion of both WL and DL processing tasks in our paradigm, we also purposely utilized expository text for our DL task. Expository text is distinct from narrative text because it conveys information on subject matter without reliance on narrative structure or characters. Key for this study, it is a genre that is increasingly relied upon during the fourth grade transition from “learning to read” to “reading to learn” and has been shown to have distinct and increased cognitive burden from narrative texts, including increased demand on processes involved in vocabulary, semantic coherence, WM, and other executive functions (Berman & Nir-sagiv, 2007; Eason, Goldberg, Young, Geist, & Cutting, 2012). Additionally, expository comprehension has been found to be more difficult for young readers, due to less global coherence markers and decreased subject background knowledge (Baretta, Tomitch, MacNair, Lim, & Waldie, 2009; Berman & Nir-sagiv, 2007). Because expository texts places increased demand on skill sets that correlate with poor RC ability, and due to its central pragmatic importance, this genre provides an ideal environment in which to examine the neural underpinnings of adolescent RC ability, and how WL and DL processes integrate. Since young readers with RC deficits struggle with overlapping but separable difficulties in both word and passage reading, we were particularly interested in the activity patterns of regions recruited for both of these tasks.
Through this paradigmatic approach, we aimed to address the following questions: 1.) What are the neural correlates of expository text comprehension, particularly in relation to WL processing regions in adolescents? and 2.) Are regions that support both word and passage reading differentiated by task-specific network connectivity patterns? For each question, we additionally sought to address how these findings might be modulated with WL and RC ability. From previous literature, we hypothesized that, as examined through GLM mean activation analyses, adolescent word and passage reading would each recruit a shared, left-lateralized processing network, including those that support orthographic (OT area), semantic functions (IFG, MTG, and TP), and potentially WM (left dlPFC). We also expected constrained recruitment of regions within the DMN specifically for DL processing. However, given the evidence that 1.) Multi-functional regions show unique predictions of a myriad of clinical outcomes, 2.) Specific language and WM regions are reported to be multi-functional across different reading demands, and 3.) Struggling readers behaviorally demonstrate multi-tier semantic and WM/executive deficits, we additionally hypothesized that semantic/WM regions activated for both passage- and word-reading were likely to underlay critical network differences (as examined through functional connectivity analyses). Importantly, we further expected that hypothesized mean activation and connectivity findings would be modulated by reading skill thus revealing novel information about reading development. Given the behavioral, theoretical, and neural implications of executive functions in RC ability, we particularly anticipated that dlPFC activation and connectivity to the language network would be associated with RC ability.
Methods
Out of an original cohort of 131 subjects who were scanned as part of a larger ongoing project of reading comprehension, we selected individuals for participation in the current study who had greater than 85 standard score IQ, and between 85–115 standard scores on basic reading tests (n = 98; see Behavioral Testing section for rationale and mean values). From these 98 subjects, subjects were excluded based on the following exclusion parameters: motion (n = 29 excluded for average motion outliers > 10%), in-scanner task performance (n = 12 excluded for poor in-scanner task performance, see below), and inadequate head coverage (n = 19). The final analysis included 38 adolescents, aged 9–14 years old (mean age = 12.1 +/− 1.5; 24 female).
All participants were native English speakers with normal hearing and vision, and no history of major psychiatric illness or traumatic brain injury/epilepsy. All subjects had no history of a developmental disability or contraindication to MRI. Each participant gave written consent at the beginning of the study, with procedures carried out in accordance with Vanderbilt University’s Institutional Review Board. Participants received $150 for behavioral and neuroimaging testing.
Behavioral Testing
Participants who met pre-screening eligibility requirements completed a comprehensive test battery (measures relevant to the current study reported in Table 2). All participants had typical IQ (standard score > 85 on Full Scale, Performance, and Verbal IQ of the Wechsler Abbreviated Scale of Intelligence; mean IQ = 107.6 +/− 8.1; Wechsler, 2011). Additionally, to ensure that participants had at least the entry level word recognition/decoding ability to complete the paradigm, participants had to have a standard score of 85–115 on the basic reading composite score and subtests of the Woodcock Mastery Test-Revised (Woodcock, 1998). Subtests of the basic reading composite score included Letter-Word Identification and Word Attack, which measure word recognition and decoding ability, respectively. As one primary question in this paper is how the full range of RC ability influences neural networks of reading, RC ability, as measured by the Gates MacGinitie (MacGinitie, 2000), was allowed to vary (mean percentile = 61.7 +/− 23.6; see Table 2 for demographic information). One subject did not complete the Gates MacGinitie, and subject’s score was replaced with the group mean for all related analyses. For the Supplemental Analysis, Sentence Span (Swanson, Cochran, & Ewers, 1989) was used to assess working memory capacity.
Table 2.
Demographic data for n = 38 subjects.
| Measure | Mean (SD) | Range |
|---|---|---|
| Age | 12.1 (1.5) | 9–14 |
| WA %ile | 47.0 (17.8) | 18–90 |
| LWID %ile | 56.2 (18.8) | 20–92 |
| Gates %ile | 61.7 (23.6) | 14–98 |
| WASI ss | 107.6 (8.1) | 89–123 |
fMRI Tasks
Passages condition (see Figure 1a)
Figure 1.
Sample stimuli from each of the three conditions.
Eight expository passages were constructed in-lab and equated across measures of word concreteness, syntactic simplicity, referential cohesion, causal cohesion, and narrativity (i.e., the degree to which the text uses everyday oral conversation and tells a story with familiar characters, events, places, and things) using Coh-Metrix 2.0 (McNamara et al., 2005). Passages were additionally matched on descriptive factors, including: number of words, average sentence length, and Flesch-Kincaid grade-level (range from 4.0–4.9), ultimately matching across 23 discourse measures. To ensure equivalence of all measures across passages, measures for each of the 8 passages were individually compared to the mean of the remaining 7 passages. Passages were considered equivalent when measures were within a 90% confidence interval of the mean of the remaining passages. Four of these passages were used for the Passages condition and four were used for the Words condition (see below), which included words from the passages in randomized order.
All passages were 150 words in length. Each sentence was no longer than 13 words. The passages were all expository and included the following topics: Hang Gliding, Wrasses, Velvet Worms, and Hydroponics. Each passage consisted of two paragraphs, the first of which served to introduce the topic while the second elaborated on a particular detail of the subject matter.
Words Condition (see Figure 1b)
The words condition consisted of scrambled words presented in “phrases”, which were exactly matched in length, word type, and presentation time to the phrases in the passages (see Figure 1b).
Symbolic Baseline (see Figure 1c)
The baseline condition included three non-alphanumeric symbols (two symbol types) displayed horizontally on a slide (see Figure 1c), and was matched in presentation time to the word and passage phrases.
Procedure
Single word presentations in sentential context have been reported to create an uncomfortable, artificial reading experience (Rayner, 1986). In the current study, passages were consequently divided into syntactic phrases (verb, noun, and propositional), ranging from 1–6 words in length. Each phrase was presented on a separate trial. We allowed 550 ms for each content word and 275 ms for each function word. For timing purposes, we presented no more than three content words per slide. The interval between phrase stimuli was randomized to allow for event-related analyses (not included in this study; jitter ranging from 550 ms-4000 ms). The baseline condition was presented between paragraph 1 and paragraph 2 of both the Passages and Words conditions. The purpose of this design was to allow participants’ activation to return to baseline after reading each block (paragraph). A typical presentation sequence was: 1) Passage condition, Paragraph 1; 2) Baseline condition; 3) Passage condition, Paragraph 2; 4) Baseline condition; 5) Words condition; 6) Baseline condition. In half of the runs per subject, the Words condition was presented first. Two lists were used, which randomly alternated whether the first run of the scanning session was Words or Passages. The mean time for the Passages block was 78.54 s (SD = 22.94); Words mean = 82.45 s (SD = 3.29); and Baseline mean = 47.69 s (SD = 1.48).
To monitor whether participants attended to all stimuli, 8% of the stimuli within each task block were randomly repeated on two consecutive screens. Participants pressed a button with their right thumb when they detected a phrase repetition or a symbol configuration repetition. Only subjects who responded to greater than 75% repetitions correctly per block and had less than 95% sporadic buttons pushes (button pushes during non-repeated stimuli), were included in the analysis.
fMRI Data Acquisition, Preprocessing and First-Level Analyses
Imaging was performed on a Philips Achieva 3T MR scanner with an 8-channel head coil. Functional images were acquired using a gradient echo planar imaging sequence with 40 (3mm thick) slices with no gap and consisted of 4 runs, each 7 minutes (190 dynamics per run). Additional imaging parameters for functional images included TE=30 msec (for optimal BOLD contrast at 3T), FOV 240 × 240 × 120 mm, slice thickness=3 mm with 0 mm gaps, 75 degree flip angle, TR=2200 msec, and a matrix size 80×80 (interpolated), yielding 3mm3 isotropic voxels.
All functional data were analyzed using MATLAB (Mathworks, Natick MA) and SPM8 (Frackowiak, Friston, Frith, Dolan, & Mazziotta, 1997). The functional data for each participant were slice-timing corrected, aligned to the mean functional image, normalized to MNI space and spatially smoothed with an 8 mm FWHM Gaussian filter. All subject masks underwent dual-rater quality assessment checks. Due to differences in subject masks, the cerebellum was not included in our analysis. In our first-level analysis, standard regression models were created using an estimated HRF for each condition; the size motion parameters (x, y, z translational; x, y, z rotational) and outlying volumes as determined by ART (Whitfield-Gabrieli; http://www.nitrc.org/projects/artifact_detect/) were included in the design matrix as regressors of no interest. Subjects with greater than 10% average motion outliers and greater than 20% motion outliers in any individual run were not included in the final analysis. For the standard GLM analyses three sets of contrasts for each participant were created: Words vs. Symbols, Passages vs. Symbols, and Passages vs. Words.
Group-Level Imaging Analysis
SPM8 and MATLAB (Mathworks, Nattick MA) were used to create whole brain activation maps. Individual contrast maps were brought up to a group level, one-sample t-test to analyze Passages and Words. The conjunction of Passages > Symbols and Words > Symbols was performed using SPM’s minimum t-value conjunction algorithm. AFNI’s 3dClustSim algorithm was used to determine the probability of false positive clusters (and appropriate correction for multiple comparisons) through iterative Monte Carlo simulations (n = 10,000). All group-level analyses were subjected to an uncorrected statistical threshold of p < 0.005 and a cluster size of 118 voxels, which was determined by 3dClustSim to be equivalent to p-corrected < .05 (2-sided).
Connectivity Analysis
Connectivity analysis was performed using the CONN toolbox (Whitfield-Gabrieli & Nieto-Castanon, 2012). The toolbox uses the CompCor method (Behzadi, Restom, Liau, & Liu, 2007) to estimate confounding signals. White matter and CSF signal (derived from T1 images; characterized by 5 dimensions), movement artifacts, six movement parameters (as determined by ART), and the first temporal derivative of the movement parameters were regressed out of the signal. To remove correlations driven by general, task-related co-activations, task effects and their first temporal derivative were also removed from the signal (Whitfield-Gabrieli & Nieto-Castanon, 2012). Analysis was run across the whole duration of the concatenated blocks per task. High-pass filtration with a cut-off value of .008 Hz was applied to remove slow oscillations driven by physiological noise. One subject was excluded from connectivity analysis due to excessive motion specifically during the T1 scan.
For subject-level analysis, the corrected voxel time-series was extracted for each pre-defined ROI (defined below), then averaged to produce one time series per ROI. Whole-brain bivariate correlation maps were then generated for each ROI and converted to Fischer’s z scores. For group-level analyses, ANCOVA models were run to identify whole-brain task-related difference with and without additional covariates of interest. For all connectivity results, only positive correlations were investigated.
Seed regions
For the connectivity analyses, we were specifically interested in isolating semantic, orthographic, and executive function regions that were active in both Words > Symbols and Passages > Symbols (Table 5). Specifically, we were interested in overlap nodes previously identified as part of the primary frontal-temporal semantic network, namely the left IFG (BA 44, 45, and 47), left MTG (BA 21), and left TP (BA 38), which have been found to support word and text-level semantic processes (Friederici, 2011; Hagoort & Indefrey, 2014; Jefferies, 2013; Price, 2012; Binder, Desai, Graves, & Conant, 2009). Additionally, we examined the pVWFA and dlPFC overlap areas, since their associated functions of orthographic-semantic and WM processes, respectively, have been implicated in unique word and text-level functions (Christopher et al., 2012; Rimrodt et al., 2009). To isolate these specific overlap regions, the Words > Symbols and Passages > Symbols conjunction (described above) was masked by Brodmann Area (BA) using the WFU PickAtlas Talairach Daemon atlas regions (WFU Pickatlas, version 2.5.2; Maldjian et al., 2003, Lancaster et al., 2000; Lancaster et al., 1997), with dilation = 3. Seeds were closely evaluated to ensure there were no overlapping voxels. As the pVWFA does not have an associated BA, the Passage and Word conjunction map was masked with a spherical ROI (radius = 10) centered at [-43, −55, −17], which was implicated as the central pVWFA point in a meta-analysis (converted to MNI; Richlan, Kronbichler, & Wimmer, 2009). Consequently, the following seeds from the Passage/Word conjunction map were run in a whole-brain connectivity analysis: IFG (comprised of BA 44, 45, and 47), MTG (BA 21), TP (BA 38), dlPFC (BA 46 and 9), and pVWFA (ROI centered at −43, −55, −17 with a radius of 10). With BA masking, the resulting dlPFC seed localized to the frontal border of BA 46/9 at the inferior frontal sulcus. In order to examine a more constrained, centralized dlPFC seed, we masked the Passage/Word conjunction map with a dlPFC map defined in the Neurosynth cortical dlPFC meta-analysis (Yarkoni et al., 2011). This seed overlapped with our original BA 46/9 seed. For all subsequent analyses of the BA 46/9 seed, we performed supplemental analysis on this secondary seed to examine the regional specificity of results.
Table 5.
Center of mass (CM) and peak coordinates for five connectivity seeds. Seeds were pulled from the Passages > Symbols and Words > Symbols GLM conjunction analysis in language and executive areas.
| Anatomical Region |
CM MNI Coordinates |
Peak MNI Coordinates |
k | BA | ||||
|---|---|---|---|---|---|---|---|---|
| x | y | z | x | y | z | |||
| L dlPFC | −52 | 18 | 28 | −44 | 16 | 26 | 116 | 46, 9 |
| L IFG | −48 | 25 | 5 | −46 | 28 | 2 | 1841 | 47, 45, 44 |
| L TP | −48 | 15 | −21 | −54 | 18 | −8 | 210 | 38 |
| L MTG | −58 | −25 | −8 | −58 | −34 | −2 | 1195 | 21 |
| L pVWFA | −43 | −55 | −18 | −44 | −48 | −14 | 489 | 37 |
For each seed, hierarchical contrasts were run for Words > Symbols and Passages > Words. For comparison purposes, Passages > Symbols, Passages alone (without baseline), and Words alone were additionally run to assess whether patterns were only due to relative differences to the baseline tasks.
GLM Results
Words > Symbols (Table 3; Figure 2)
Table 3.
GLM analyses for Words > Symbols, Passages > Symbols, and Passages > Words. Cluster size (k) in mm^3. BA, Brodmann Area. All T-values are significant at p = 0.05. For large clusters, brackets indicate sub-cluster peaks in BA or functional regions distinct from primary peak, extracted using a decreased peak search space of 4 mm within the main cluster. Clusters are identified by general cognitive domains based on the majority of sub-peak locations within the cluster.
| GLM Contrast |
Anatomical Region | MNI Coordinates |
k | Max T | BA | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
|
Words > Symbols | |||||||
| Language and WM |
L Middle Occipital | −24 | −100 | −4 | 26997 | 17.99 | 18 |
| R Middle Occipital | 22 | −100 | −6 | [ ] | 15.63 | 18 | |
| L Inferior Occipital | −38 | −86 | −12 | [ ] | 10.05 | 19 | |
| L Fusiform | −40 | −44 | −26 | [ ] | 9.71 | 37 | |
| L pVWFA | −46 | −48 | −12 | [ ] | 9.41 | 37 | |
| R Cuneus | 2 | −86 | 26 | [ ] | 8.18 | 17 | |
| L IFG | −46 | 30 | −4 | [ ] | 8.08 | 45, 47, 44 | |
| L MTG/STG | −52 | −42 | 4 | [ ] | 7.83 | 22 | |
| L Hippocampus | −24 | −30 | −4 | [ ] | 7.77 | 35 | |
| L Cuneus | −2 | −92 | 22 | [ ] | 7.31 | 17 | |
| L MTG | −50 | −34 | 0 | [ ] | 6.86 | 21 | |
| L Precentral Gyrus | −52 | −2 | 50 | [ ] | 6.3 | 6 | |
| L anterior MTG: TP | −58 | −12 | −14 | [ ] | 6.28 | 21/38 | |
| L ventral IFG | −52 | 20 | 2 | [ ] | 6.12 | 47 | |
| L dorsal IFG: dlPFC | −46 | 12 | 24 | [ ] | 5.69 | 45, 46, 9 | |
| L TP | −44 | 20 | −18 | [ ] | 5 | 38 | |
| R Fusiform Gyrus | 38 | −50 | −22 | [ ] | 4.59 | 37 | |
| R ventral IFG: TP | 54 | 40 | −10 | 1302 | 5.39 | 47 | |
| R IFG | 40 | 24 | 22 | 156 | 3.64 | 45 | |
| Memory and Motor |
R Hippocampus: Thalamus |
24 | −28 | −4 | 1186 | 7.27 | 35 |
| L SMA | −2 | 2 | 68 | 142 | 4.46 | 6 | |
|
Passages > Symbols | |||||||
| Language, Memory, and Executive |
L IFG | −48 | 30 | 2 | 29810 | 11.61 | 45, 47 |
| L ventral IFG | −48 | 28 | −10 | [ ] | 11.28 | 47 | |
| L Middle Occipital | −22 | −102 | −4 | [ ] | 11.03 | 18, 17 | |
| L MTG | −58 | −34 | −2 | [ ] | 10.48 | 21 | |
| R Calcarine | 12 | −90 | 4 | [ ] | 10.39 | 17 | |
| L Fusiform: Hipp. | −42 | −46 | −26 | [ ] | 9.49 | 37 | |
| L pVWFA | −50 | −46 | −10 | [ ] | 9.33 | 37 | |
| L IFG | −46 | 16 | 22 | [ ] | 9.24 | 44, 45 | |
| L STG: AG | −64 | −42 | 2 | [ ] | 9.06 | 22, 39 | |
| L Precentral | −48 | 4 | 48 | [ ] | 8.48 | 6 | |
| L dorsal IFG: dlPFC | −38 | 8 | 28 | [ ] | 7.96 | 45, 9 | |
| L TP | −50 | 10 | −28 | [ ] | 7.24 | 38, 21 | |
| L MFG/SFG | −10 | 50 | 48 | [ ] | 6.78 | 8, 9 | |
| R STG/MTG | 46 | −34 | 0 | 2605 | 6.52 | 21 | |
| R ITG | 66 | −44 | −16 | [ ] | 5.8 | 21, 20 | |
| R TP | 48 | 18 | −30 | [ ] | 5.33 | 38 | |
| R IFG | 60 | 30 | 12 | 2732 | 8.19 | 46, 45 | |
| R Precentral Gyrus | 62 | −8 | 42 | [ ] | 6.19 | 6 | |
| R MFG | 56 | 22 | 38 | [ ] | 4.83 | 9 | |
| R MFG | 42 | 38 | −16 | [ ] | 4.8 | 11 | |
| R Insula | 48 | −14 | 18 | [ ] | 4.73 | 43 | |
| R ventral IFG | 54 | 32 | −12 | [ ] | 4.06 | 47 | |
| R AG: MTG | 54 | −70 | 30 | 515 | 5.42 | 39 | |
| R Parahipp/Hipp | 26 | −26 | −8 | 165 | 7.22 | 35 | |
|
Passages > Words | |||||||
| DMN | L AG | −44 | −60 | 30 | 1286 | 4.92 | 39 |
| L MTG/anterior STS | −50 | −24 | −10 | 1045 | 5.4 | 21, 20 | |
| R MTG: AG | 52 | −76 | 16 | 884 | 5.14 | 39, 19 | |
| R Superior Occipital | 40 | −86 | 26 | [ ] | 4.45 | 19 | |
| L/R PCU | 0 | −56 | 34 | 380 | 4.29 | 7, 31 | |
| R MTG/anterior STS | 54 | −2 | −26 | 251 | 4.49 | 21, 20 | |
| L SFG | −20 | 26 | 52 | 180 | 3.41 | 6 | |
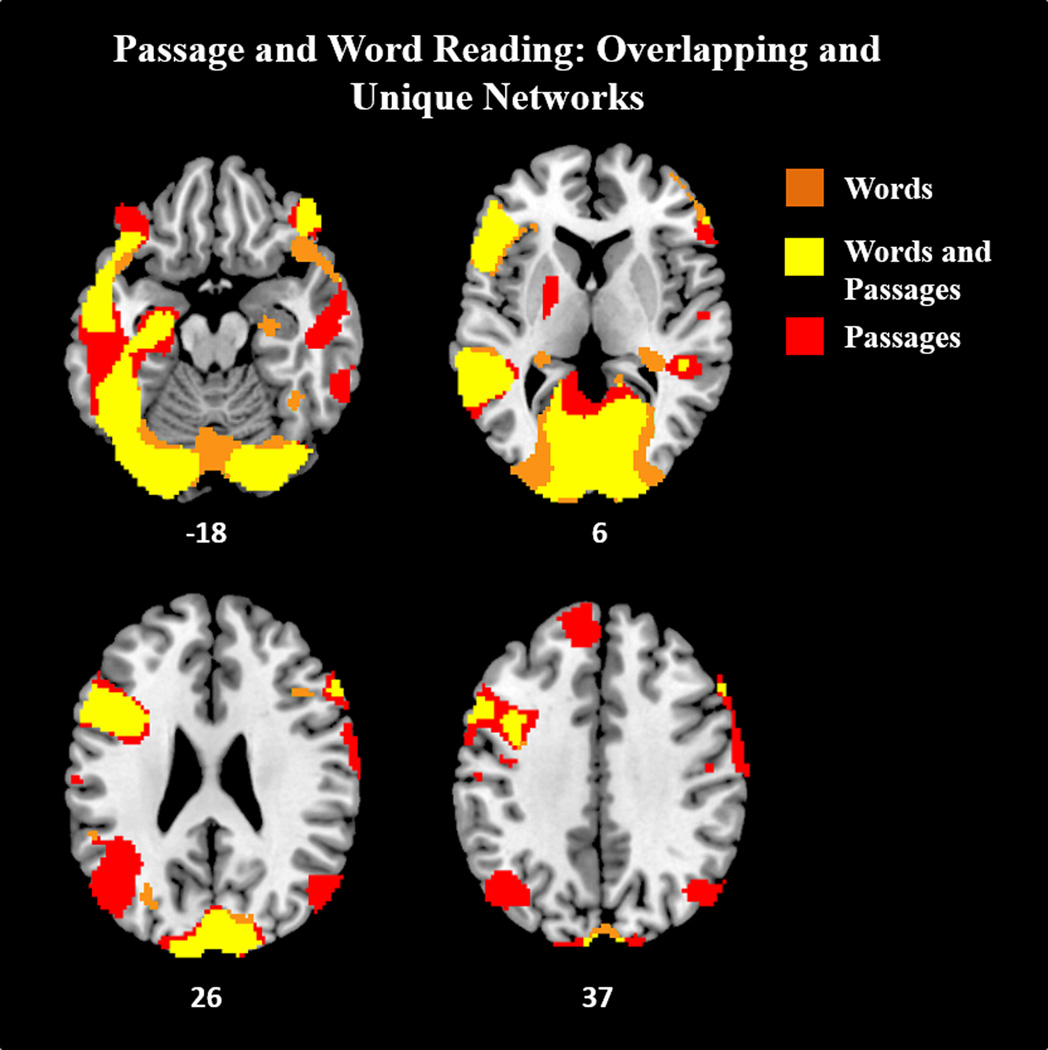
Figure 2.
A Boolean rendering of Passages > Symbols and Words > Symbols show that both Passagae reading and Word reading activate a dispersed, overlapping language and WM network. Results displayed at p-corrected <.05 (p-unc < .005, k=118).
Compared to Symbols, Word reading elicited greater activation in language areas and language homologues including left fusiform gyrus (including pVWFA), bilateral/left dominant IFG (BA 47, 44, 45) extending bilaterally into dlPFC (BA 46/9), bilateral MTG, left STG extending to left ventral SMG, and bilateral temporal poles. Additional activations included motor regions (SMA and left precentral), bilateral hippocampus, left putamen, bilateral anterior insula, and bilateral occipital regions extending into ITG and MTG.
Passages > Symbols (Table 3; Figure 2)
Compared to Symbols, Passage reading exhibited greater activation in traditional perisylvian language areas and their right hemisphere homologues. These included pVWFA, bilateral/left dominant IFG (BA 47, 44, 45), bilateral MTG, and bilateral TP. Left and right IFG additionally extended upwards into the dlPFC (BA 46/9). Regions associated with the DMN were also seen, including bilateral/left dmPFC/SFG extending to SMA, bilateral AG, PCU, bilateral STS, and bilateral hippocampus. Additional activations were seen in left putamen, bilateral dorsal insula/rolandic operculum, bilateral occipital regions extending into ITG and MTG, and bilateral precentral gyrus.
Passages > Word Reading (Table 3; Figure 3)
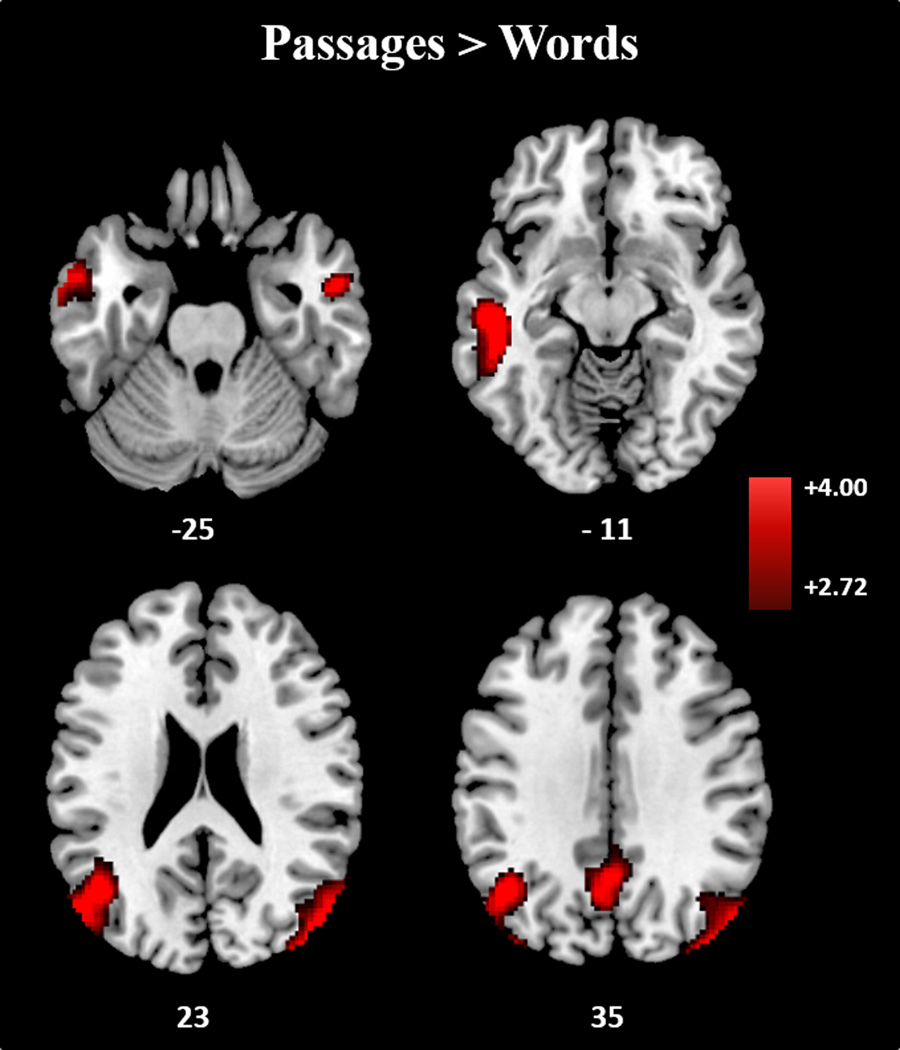
Figure 3.
Expository text comprehension, as compared to WL reading, uniquely recruits regions in the DMN, including bilateral AG, PCC, and bilateral anterior STS. Results displayed at p-corrected <.05 (p-unc <.005, k = 118).
In a direct contrast of Passages vs. Words, Passages showed greater activation in heteromodal regions, including bilateral/left dominant TP extending to left MTG, bilateral anterior superior temporal sulcus (STS), bilateral AG, and dorsal PCU. Except for dorsal PCU, all of these activations were also greater in Passage-Baseline.
Word > Symbols Modulated by Word Reading Ability (Table 4; Figure 4)
Table 4.
GLM covariate for word recognition (LWID) and decoding (WA) ability (in Words > Symbols) and RC ability (Gates percentile; Passages > Symbols). Cluster size (k) in mm^3. BA, Brodmann Area. All T-values are significant at p = 0.05. For large clusters, brackets indicate sub-cluster peaks in BA or functional regions distinct from primary peak, extracted using a decreased peak search space of 4 mm within the main cluster. Clusters are identified by general cognitive domains based on the majority of sub-peak locations within the cluster.
| Covariate | Anatomical Region | MNI Coordinates |
k | Max T | BA | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
|
Words > Symbols | |||||||
| LWID | |||||||
| Language | L Fusiform/OT area | −44 | −46 | −12 | 140 | 3.89 | 37 |
| L MTG/STG | −46 | −50 | 4 | 452 | 4.4 | 21, 22 | |
| L IFG | −52 | 22 | 20 | 173 | 3.35 | 45, 44 | |
| L IPL/SPL | −28 | −50 | 46 | 175 | 3.79 | 40 | |
| WA | |||||||
| Language | L anterior MTG/STG | −54 | −16 | −8 | 255 | 4.83 | 21, 22, 38 |
| L posterior MTG/STG | −44 | −52 | 8 | 156 | 3.69 | 21, 22, 39 | |
|
Passages > Symbols | |||||||
| Gates McGinitie | |||||||
| Language and WM | L IFG: dlPFC | −56 | 22 | 14 | 2308 | 6.36 | 45, 47, 44, 9 |
| R TP: ventral IFG | 30 | 22 | −26 | 2050 | 5.31 | 47, 38 | |
| L TP | −44 | 6 | −42 | [ ] | 4.98 | 38 | |
| L anterior MTG | −56 | −18 | −20 | 810 | 4.39 | 21 | |
| L fusiform | −58 | −50 | −12 | [ ] | 4.15 | 37 | |
| DMN | L SFG | −10 | 40 | 50 | 3875 | 6.08 | 8 |
| L SFG | −8 | 26 | 54 | [ ] | 5.07 | 6 | |
| L PCU | −6 | −52 | 40 | 561 | 5.32 | 7 | |
| L PCC | −8 | −54 | 24 | [ ] | 3.06 | 31 | |
| L AG | −46 | −64 | 32 | 1538 | 4.96 | 39 | |
| R AG | 54 | −66 | 38 | 119 | 3.34 | 39 | |
| L Hippocampus | −20 | −6 | −18 | 865 | 6.48 | 34 | |
| Motor and Visual | R Lingual /Cuneus | 2 | −86 | 0 | 471 | 4.55 | 18, 17 |
| R Postcentral | 38 | −30 | 48 | 394 | 4.06 | 40, 3, 2 | |
| L Thalamus | −6 | −14 | 8 | 185 | 4.03 | NA | |
| R Caudate | 14 | 10 | 14 | 164 | 3.85 | NA | |
| R SPL | 18 | −68 | 64 | 192 | 3.77 | 7 | |
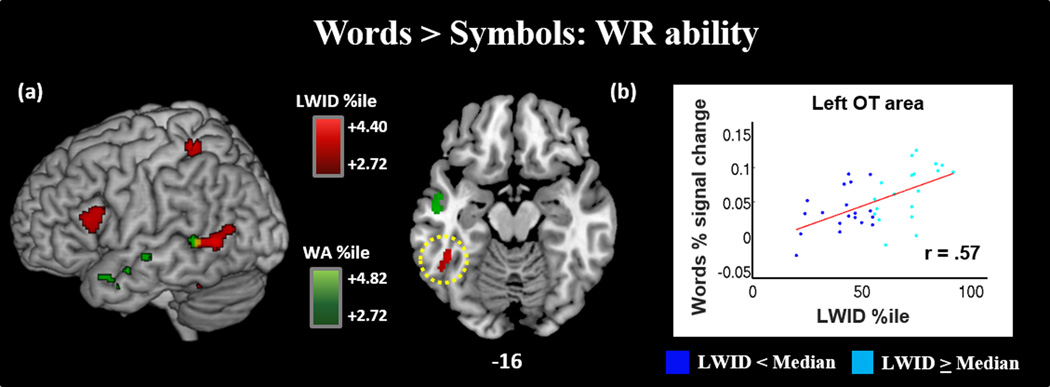
Figure 4.
(a) During Word reading only, word reading ability, as measured by Word Attack (green) and Letter Word Identification (red) measures, predicts activation in language regions, including the orthographic processing regions in the left OT area. Both measurements of word reading predicted activation in the left MTG (yellow). (b) Plot of LWID Percentile score by Words percet signal change in the left OT area. Low and high word reading ability (as determined by median split of LWID percentile) represented in dark blue and light blue, respectively. Results displayed at p-corrected <.05 (p.unc <.005, k=118).
In Word reading greater than Symbols, both Word Attack (WA) and Letter Word ID (LWID) show positive correlation with activation of the left MTG. Each measurement also predicted activation in unique regions:
Word > Symbols Modulated by Word Attack (WA) (Table 4; Figure 4a): WA percentile showed unique positive correlations with activation in the left anterior STS, extending into left TP.
Word > Symbols Modulated by Letter Word Identification (LWID) (Table 4; Figure 4a): LWID percentile showed unique positive correlations with activation in the left OT area (including pVWFA), left IFG, and left superior parietal lobule.
Passages > Symbols Modulated by Gates Percentile (Table 4; Figure 5)
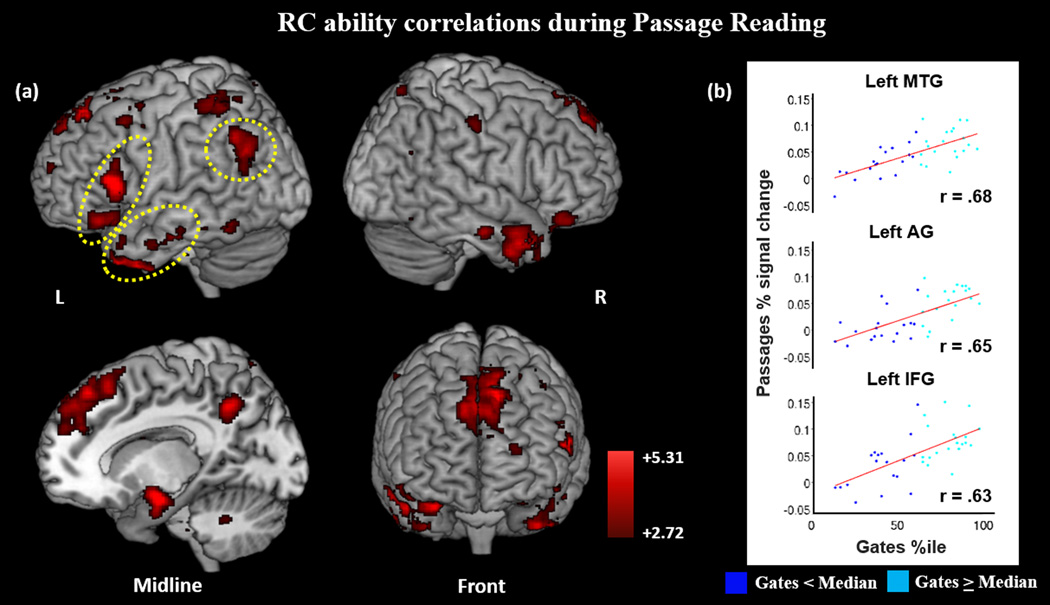
Figure 5.
(a) During Passage versus Baseline, RC ability predicts activation in both language and EF regions, including areas that support WM and the DMN. (b) Selected plots of Gates percentile by Passage percent signal change in L MTG, L IFG, and L AG (circled in yellow on (a)). Low and high RC ability (as determined by median split of Gates percentile) represented in dark blue and light blue, respectively. Results displayed at p-corrected <.05 (p-unc <.005, k=118).
In Passages greater than Symbols, Gates percentile was positively correlated with activation in left IFG (BA 47, 45, and 44) extending into left insula, left dlPFC (BA 46 and BA 9), bilateral TP extending into right ventral IFG and ventral insula, and pVWFA. Additional results included positive correlation between Gates and activation in regions associated with the DMN, including dmPFC, bilateral AG, dorsal PCU, PCC, anterior cingulate cortex (ACC), left hippocampus extending to the amygdala, and bilateral anterior STS. Additional correlations were seen in bilateral postcentral, right caudate, left thalamus, right SPL, and bilateral lingual gyrus.
Connectivity Results
Seed-to-Whole-Brain Connectivity of Overlap Regions (positive correlations only)
We ran whole-brain analysis on the 3 language seeds (L IFG, L MTG, and L TP), pVWFA, and dlPFC GLM conjunction areas (activations seen in both Passages > Symbols and Words > Symbols). All results are reported at p < .05 as determined by 3dClustSim (p-uncorr < .005, k = 118). To identify shared correlations across seeds, seed-to-whole-brain analyses were run separately for each seed region, and additional Boolean overlap maps were generated across seed correlation maps to identify areas of convergent correlations. Results that fall within the language and WM network are in bold. Seed region characteristics are reported in Table 5.
Words > Symbols (Table 6)
Table 6.
Seed-to-whole-brain connectivity analyses for Words > Symbols. Overlap center of mass coordinates are reported in final rows. Cluster size (k) in mm^3. BA, Brodmann Area. All T-values are significant at p < 0.05.
| Seed region |
Whole-Brain Correlation Regions |
MNI Coordinates |
k | Max T | BA | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
|
Words > Symbols | |||||||
| L IFG | R IFG | 50 | 20 | 30 | 1456 | 5.86 | 44, 45, 46, 9, 8 |
| R/L Middle Occipital: L OT area |
42 | −84 | −4 | 4106 | 5.46 | 18, 37 | |
| L Insula | −34 | 10 | 20 | 366 | 5.11 | 13, 44 | |
| R Postcentral | 12 | −44 | 70 | 420 | 4.45 | 3, 7 | |
| R Orbitofrontal/IFG | 42 | 40 | −12 | 284 | 4.44 | 11, 47 | |
| L Postcentral | −60 | −6 | 40 | 238 | 4.31 | 6, 9 | |
| L Postcentral | −56 | −6 | 16 | 140 | 3.97 | 43, 4 | |
| L TP | L Insula/RO | −36 | 8 | 20 | 180 | 5.24 | 13, 44 |
| R OT area | 52 | −50 | −24 | 259 | 4.43 | 37 | |
| L OT area | −42 | −72 | −10 | 276 | 4.30 | 37 | |
| R Precentral | 14 | −32 | 72 | 208 | 4.10 | 3 | |
| R IFG | 46 | 18 | 14 | 224 | 3.79 | 44 | |
| L MTG | L Postcentral | −64 | −6 | 20 | 471 | 5.13 | 4, 43, 44 |
| R Middle Occipital | 44 | −84 | −10 | 775 | 5.12 | 18, 19 | |
| L Middle Occipital: L OT area |
−34 | −86 | −4 | 620 | 4.68 | 18, 19 | |
| L Paracentral Lobule | −14 | −30 | 70 | 173 | 4.39 | 4 | |
| R Paracentral Lobule | 14 | −38 | 64 | 434 | 4.20 | 4 | |
| pVWFA | L MTG | −46 | −40 | 2 | 566 | 4.82 | 21, 22 |
| L Middle Occipital | −26 | −84 | 6 | 457 | 4.36 | 18 | |
| L MFG | −46 | 4 | 52 | 294 | 4.33 | 6 | |
| R Middle Occipital | 30 | −96 | 2 | 187 | 4.05 | 18 | |
| L Insula | −36 | 10 | 20 | 126 | 4.03 | 13, 44 | |
| R Postcentral | 20 | −24 | 52 | 221 | 3.89 | 4 | |
| L dlPFC | L MTG | −48 | −38 | 4 | 138 | 4.52 | 21 |
| L Middle Occipital | −20 | −106 | −2 | 150 | 4.23 | 18 | |
| R Middle Occipital | 14 | −102 | 16 | 531 | 4.17 | 18 | |
| L MFG | −58 | 12 | 34 | 154 | 4.12 | 9, 6 | |
| R MFG/Precentral | 46 | 8 | 30 | 461 | 3.97 | 9, 46 | |
| L Rolandic Operculum | −44 | −6 | 16 | 250 | 3.89 | 13 | |
|
Overlap Regions | |||||||
| pVWFA | −41 | −55 | −16 | 190 | − | 37 | |
| L MTG | −49 | −40 | 3 | 121 | − | 21, 22 | |
3 Language Seeds (L IFG, L MTG, L TP; Figure 6): In Words compared to Symbols, all language seeds correlated with left OT area extending into pVWFA. Regions also correlated with bilateral middle occipital areas (IFG and MTG), right IFG (IFG and TP), left frontal operculum/RO (IFG and TP), primary motor and somatosensory cortices (IFG, TP, and MTG), and right ITG (TP). Supplemental analysis indicated that IFG correlations with the left pVWFA were driven by BA 44 and BA 47 (Supplemental Table 1; Supplemental Figure 1).
Figure 6.
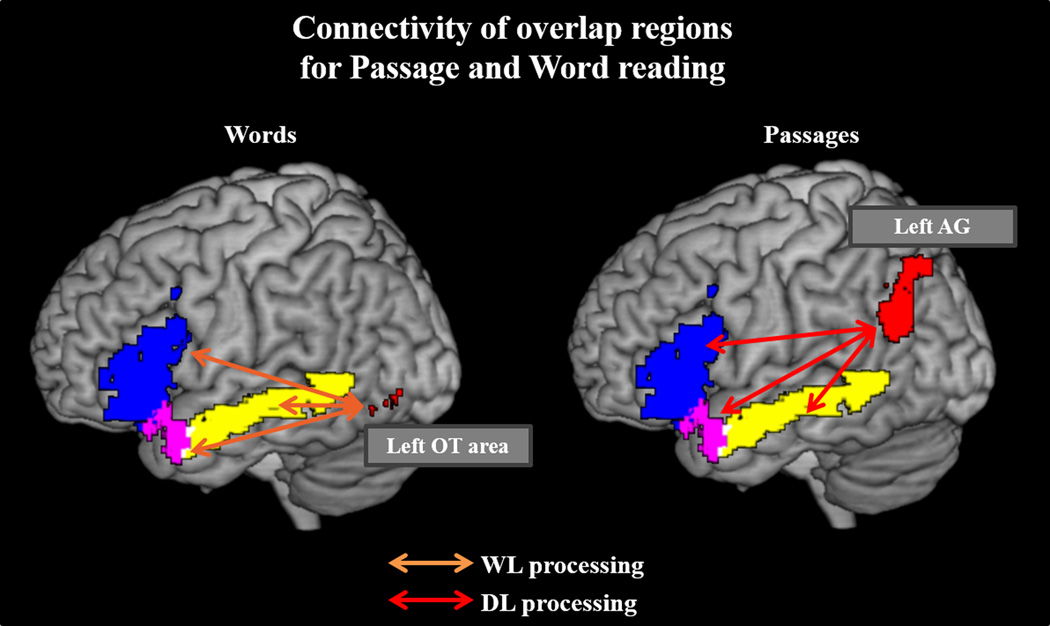
Left-lateralized language regions of mean overlap activity in Passage and Word reading show differential connectivity patterns in WL (Words > Symbols; orange arrow) and DL (Passages > Words; red arrow) processes. Specifically, the three seeds show convergent correlation with the left OT area during WL reading, and additively shows correlation with the left AG during Passage reading. Results displayed at p-corrected <.05 (p-unc <.005, k=118).
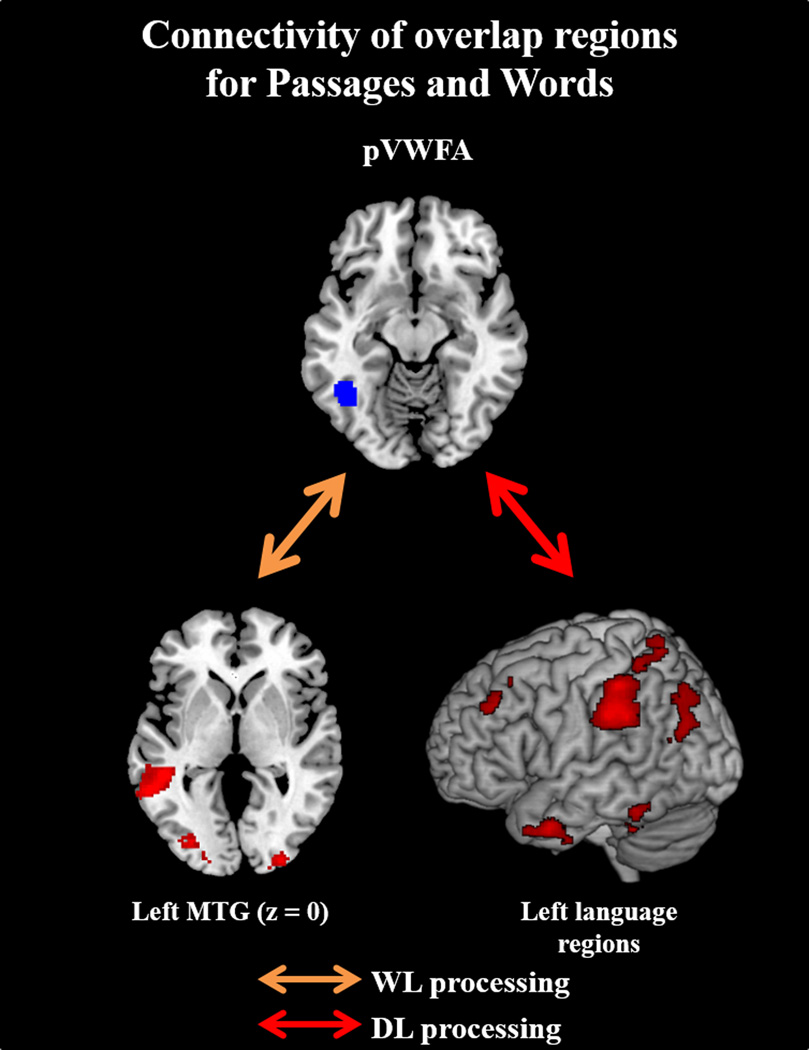
pVWFA (Figure 7): The pVWFA was more strongly correlated with left MTG in Words than Symbols, as well as with bilateral occipital regions, left insula, bilateral precentral gyrus, right postcentral, and right middle frontal gyri.
Figure 7.
Left pVWFA of mean overlap activity in Passage and Word reading shows differential connectivity patterns in WL (Words > Symbols; orange arrow) and DL (Passages > Words; red arrow) processes. During WL processes, pVWFA correlates with the left MTG and primary sensory regions. The pVWFA then additively shows correlation with the left AG, SMG, and language network/language homologues during Passage reading. Results displayed at p-corrected < .05 (p-unc <.005, k=118).
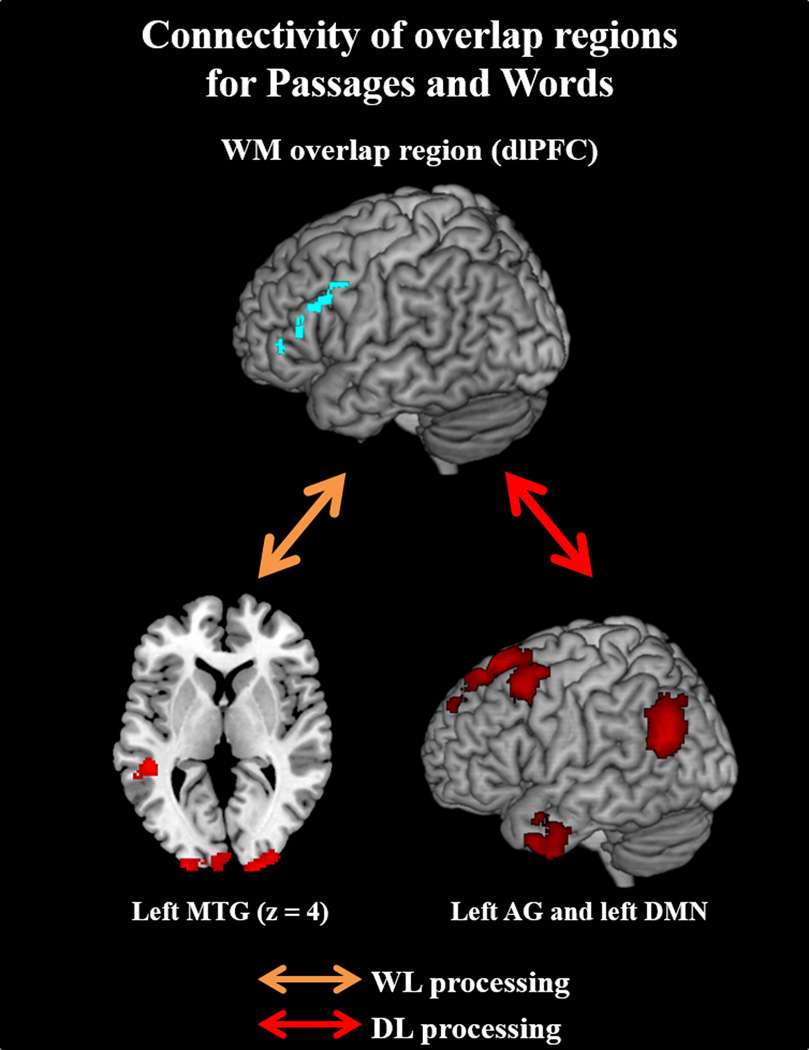
dlPFC (Figure 8): In Words compared to Symbols, dlPFC correlated with left MTG, along with right dlPFC (BA 9/46), left MFG (BA 9, 6), bilateral middle occipital regions, and left RO in Words compared to Symbols. The more constrained dlPFC seed did not replicate BA 46/9 word-level findings, instead showing connectivity to bilateral precentral, bilateral RO, right orbitofrontal, and right dlPFC.
Figure 8.
Left d1PFC of mean overlap activity in Passage and Word reading shows differential connectivity patterns in WL (Words > Baseline; orange arrow) and DL (Passages > Words; red arrow) processes. During WL processes, dlPFC correlates with the same left MTG area seen in the pVWFA seed connectivity analysis. The d1PFC then additively shows correlation with the left AG and left DMN during Passage reading. Results displayed at p-corrected <.05 (p-unc <.005, k=118).
Passages > Words (Table 7)
Table 7.
Seed-to-whole-brain connectivity analyses for Passages > Words. Overlap center of mass coordinates are reported in final row. Cluster size (k) in mm^3. BA, Brodmann Area. All T-values are significant at p < 0.05.
| Seed Region |
Whole-Brain Correlation Regions |
MNI Coordinates |
k | Max T | BA | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
|
Passages > Words | |||||||
| L IFG | L AG | −44 | −62 | 24 | 1065 | 6.00 | 39 |
| L TP | −54 | 0 | −38 | 312 | 4.55 | 38, 21, 20 | |
| L Thalamus | −10 | −4 | 12 | 169 | 4.39 | NA | |
| L SFG | −12 | 40 | 52 | 139 | 4.35 | 8 | |
| R PCC: Cuneus | 24 | −66 | 8 | 499 | 4.17 | 30, 31, 18 | |
| R Thalamus | 8 | −6 | −2 | 255 | 4.13 | NA | |
| L TP | L PCU | −4 | −68 | 34 | 970 | 5.82 | 7, 31 |
| R Caudate Head | 8 | 2 | 4 | 1102 | 5.32 | NA | |
| L AG | −40 | −62 | 38 | 1417 | 5.26 | 39 | |
| L SFG: ACC | −18 | 62 | 6 | 860 | 4.72 | 10, 32 | |
| L MTG | −64 | −26 | −6 | 260 | 4.62 | 21, 20 | |
| L PCC/Calcarine | −26 | −66 | 6 | 143 | 4.50 | 30 | |
| R SFG | 12 | 36 | 40 | 465 | 4.50 | 6 | |
| L Lingual Gyrus | −12 | −46 | 2 | 190 | 4.15 | 30 | |
| L MTG | L AG | −44 | −58 | 22 | 1050 | 6.51 | 39 |
| L SFG: SMA | −10 | 40 | 52 | 139 | 5.26 | 8, 6 | |
| L MTG | −64 | −44 | 2 | 225 | 4.14 | 21 | |
| L/R Lingual Gyrus | −14 | −92 | −14 | 278 | 3.99 | 17 | |
| R Calcarine | 24 | −58 | 6 | 126 | 3.88 | 18 | |
| pVWFA | L SMG | −56 | −30 | 38 | 744 | 5.25 | 40 |
| L dlPFC | −30 | 18 | 42 | 133 | 4.59 | 8, 9 | |
| L TP | −54 | 4 | −30 | 189 | 4.51 | 21, 38, 20 | |
| R TP | 48 | −2 | −32 | 126 | 4.50 | 21, 38, 20 | |
| L AG | −38 | −64 | 22 | 571 | 4.45 | 39 | |
| R Fusiform/Parahippocampal | 38 | −38 | −26 | 342 | 4.42 | 37, 20 | |
| L Cingulate | −10 | −32 | 40 | 282 | 4.40 | 31 | |
| L ITG | −58 | −46 | −18 | 227 | 4.38 | 20 | |
| L Thalamus | −6 | −26 | 12 | 127 | 4.13 | NA | |
| L dlPFC | L AG | −40 | −62 | 26 | 1222 | 6.77 | 39 |
| L SFG | −14 | 24 | 62 | 1118 | 5.44 | 9, 8, 6 | |
| R Parahippocampal | 28 | −24 | −28 | 319 | 4.85 | 36 | |
| L TP | −50 | 4 | −22 | 392 | 4.54 | 38, 21, 20 | |
| R ITG | 26 | −4 | −38 | 126 | 4.44 | 20, 36 | |
| L Thalamus | −12 | −26 | −8 | 151 | 3.49 | NA | |
| L PCC | −6 | −56 | 6 | 184 | 3.46 | 29 | |
|
Overlap Regions | |||||||
| L AG | −41 | −62 | 30 | 159 | − | 39 | |
3 Language Seeds (L IFG, L MTG, L TP; Figure 6): All language seeds correlated with left AG more strongly in Passages compared to Words. Additionally, during Passages, language seeds showed correlation with other language areas including left ventral MTG (MTG and TP) and left TP/anterior MTG (IFG), along with bilateral caudate (IFG and TP), SFG (IFG, TP, MTG; different subdivisions), occipital regions (IFG, TP, and MTG), bilateral thalamus (IFG), PCC/PCU (IFG and TP), and ACC (TP). Supplemental analysis indicated that IFG correlations with the left AG were driven by BA 45 and BA 47 (Supplemental Table 1; Supplemental Figure 1).
pVWFA (Figure 7): In Passages greater than Words, the pVWFA correlated with left AG, bilateral TP, left SMG, left thalamus, left middle cingulate, right MTG, left/bilateral fusiform, and left dlPFC/MFG.
dlPFC (Figure 8): In Passages greater than Words, the left dlPFC correlated more strongly with left AG (which overlapped with the AG seen in the 3 language seeds and pVWFA connectivity results), extending into more dorsal AG and regions of the left lateralized DMN, specifically showing greater connectivity to left-lateralized PCC, SFG, and temporal pole. Additionally, dlPFC correlated with right ventral fusiform/parahippocampal regions. With the exception of left PCC, findings were replicated with the constrained dlPFC seed, which additionally showed connectivity to dorsal PCU.
Of note, the 3 language seeds, pVWFA, and left dlPFC all showed convergent correlation with the left AG in Passages compared to Words.
Seed-to-Whole-Brain Connectivity Analysis of Overlap Regions with Reading Metrics
To assess how out-of-scanner behavioral reading measures (WA, LWID, and RC) predicted correlations among the regions of interest, we ran whole-brain connectivity from each the original seeds (3 language seeds, VWFA, and left dlPFC).
Our whole-brain connectivity results were masked by the five original seeds, as well as the seed extracted from the whole-brain connectivity analysis (L AG). This approach allowed us to constrain our findings to specific areas of interest, without excluding any potential diverse correlations with functional subregions in our areas of interest.
Words > Symbols (Table 8; Figure 9)
Table 8.
Seed-to-whole-brain connectivity analyses correlated with word reading and RC ability for Words > Symbols and Passages > Words, respectively. Reported results restricted to regions of interest in seed-to-whole-brain connectivity analyses (left IFG, MTG, and TP; pVWFA; dlPFC; L AG). Cluster size (k) in mm^3. BA, Brodmann Area. All T-values are significant at p < 0.05.
| Covariate and Seed Region |
Result | MNI Coordinates |
k | Max T | r | BA | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
|
Words > Symbols | ||||||||
| WA | ||||||||
| pVWFA | L MTG | −44 | −50 | 4 | 273 | 4.13 | 0.78 | 21, 22 |
|
Passages > Words | ||||||||
| Gates McGinitie | ||||||||
| L dlPFC | L AG | −44 | −68 | 26 | 132 | 3.83 | 0.56 | 39 |
Figure 9.
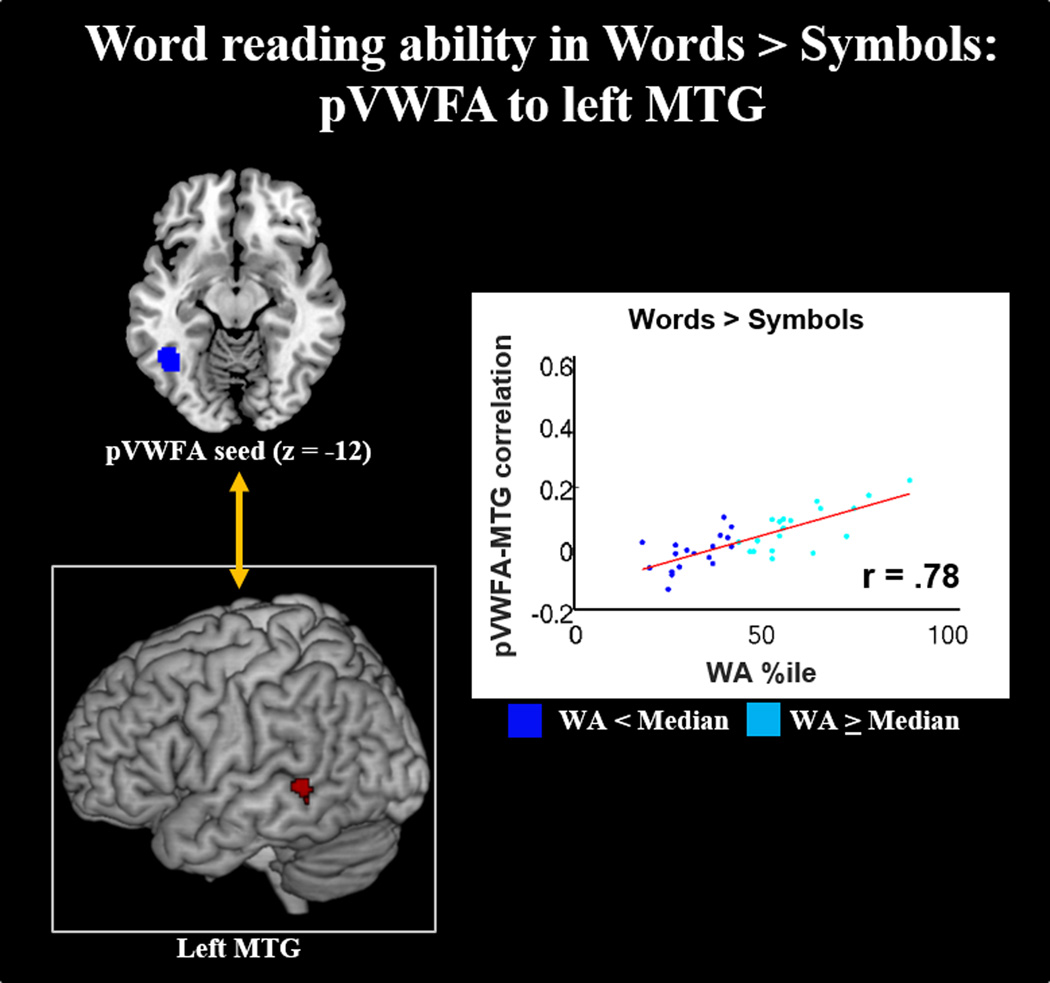
Word reading ability positively predicts correlations between left pVWFA and left MTG in WL processing. Low and high word reading ability (as determined by median split of WA percentile) represented in dark blue and light blue, respectively). Results displayed at p-corrected <.05 (p-unc <.005, k=118).
WA: In Words compared to Symbols, phonological decoding was positively correlated with increased connectivity between the left VWFA seed and left MTG. No other seed regions showed significant correlations predicted by WA percentile within the defined mask.
LWID: Word recognition did not predict correlations from any of the seed regions.
Passages > Words (Table 8; Figure 10)
Figure 10.
RC ability positively predicts correlations between the left d1PFC and the left AG in DL processing. Low and high RC ability (as determined by median split of Gates percentile) represented in dark blue and light blue, respectively). Results displayed at p-corrected <.05 (p-unc <.005, k=118)
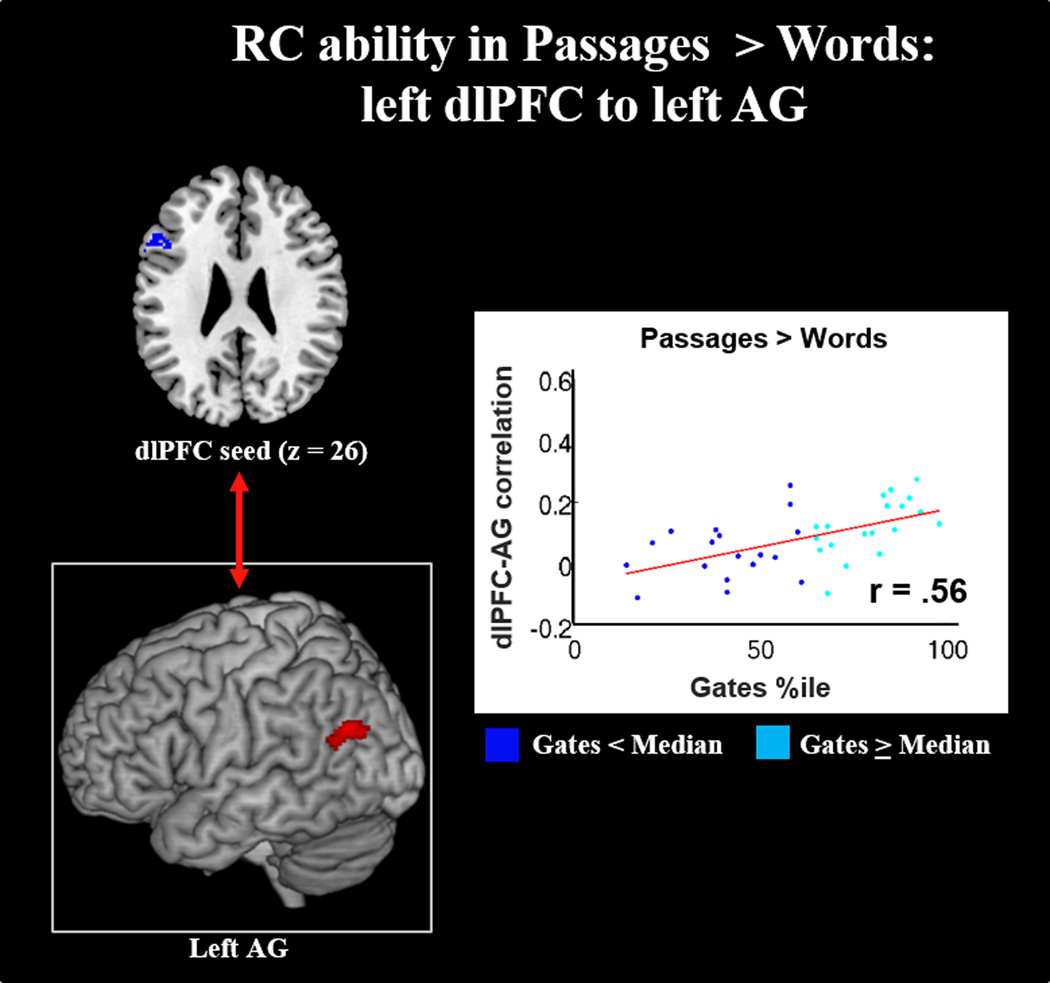
Gates: In Passages compared to Words, reading comprehension ability was positively correlated with connectivity between the left dlPFC and left ventral AG. No other seed regions showed significant correlations predicted by Gates percentile within the defined mask, including the more constrained, supplemental dlPFC seed.
Supplemental Physio-physiological Results
To assess how increased activation in dlPFC predicted whole-brain left AG results during Passage reading, the time series for the convergent left AG (from all 5 seeds) was extracted and entered into a first-level, whole-brain connectivity analysis in which each voxel-level time series was predicted by the interaction of the left AG and left dlPFC time series. Second-level t-test was run to compare the interaction term for Passages versus Symbols.
Passages > Symbols, dlPFC activation x left AG whole-brain connectivity
One unit increase in dlPFC activation predicted increased correlation between the left AG and the left VWFA, as well as the left parahippocampal gyrus. Findings were replicated in Passages alone. Preliminary analysis indicates that dlPFC prediction of left AG to the left OT area is positively correlated with WM span (see Supplemental Table 2 and Supplemental Figure 2). Findings were replicated for the constrained dlPFC seed.
Discussion
The goal of this study was to identify the neural networks that support adolescent discourse processing, and how these networks may be modulated by level of reading skill. We had two main questions: 1.) What are the neural correlates of expository passage reading, and, more centrally, how are these networks related to WL processing in adolescents? 2.) Do brain areas that are active for both word and passage reading, particularly language and WM processing regions, show separable, task-specific connectivity patterns? With each question, we also sought to understand how behavioral indices of WL reading and RC ability modulated findings.
Question 1: Neural correlates of expository text comprehension in adolescent readers
Consistent with previous work, our GLM results showed that during both word and passage reading adolescents recruited left-lateralized language areas traditionally associated with reading (see Figure 2). These include regions thought to support rapid visual word recognition (left OT areas and pVWFA) and areas associated with semantic processing (left IFG, MTG, and TP). This overlap network also included the dlPFC, a critical region in WM processes for both WL and DL processes. Additionally, there were areas uniquely associated with DL processing. As compared to WL processing, adolescent readers activated portions of the DMN, which has previously been seen in other DL analyses and is thought to support integration of world knowledge (see Figure 3; Ferstl et al., 2008; Mar, 2011).
One plausible hypothesis for the function of overlap regions between word and passage reading could be that they perform common functions (e.g., primarily underpin processes important for WL reading, since passage reading includes word processing). However, the overlapping activations across both tasks could obscure complex, task-specific processes. Previous fMRI studies suggest that these regions seen in both word and passage reading are “multi-functional” within and outside of the language domain, either through as-of-yet undefined functional subdivisions (Friederici, 2011) or functionally flexible neuronal populations (Hagoort, 2005). For instance, areas in left IFG have been found to support multiple cognitive processes: BA 45 is associated with both semantic and syntactic unification (Hagoort & Indefrey, 2014; Hagoort, 2005; Price, 2012), and BA 44 has been found to support phonological, syntactic, and speech-motor mapping functions (Fadiga et al., 2006; Friederici, 2011; Amunts, 2012). TP is implicated in semantic memory and domain-general meaning associations across stimulus modalities (Tsapkini et al., 2011), and the pVWFA has been proposed to be involved in general visual processes which include but are not limited to a role in word identification (Vogel, Petersen, & Schlaggar, 2014). While MTG is primarily studied in the context of language, within this domain it is associated with word and text-level processes, including syntax (Hagoort, 2014), semantic storage (Price, 2012), and semantic control (Jefferies, 2013; Whitney, Kirk, O’Sullivan, Lambon Ralph, & Jefferies, 2011). In this context, the literature therefore encourages an exploration of these regions in the context of their flexible “information processing characteristics” (Vogel et al., 2014) rather than restrictive cognitive properties.
This movement towards identifying regions based on information processing characteristics has been more successfully accomplished in domain-general areas, such as our final overlap region of interest, the left dlPFC. The dlPFC has been found to support a range of higher-level functions, including working memory (WM) and top-down executive control (Ptak, 2012), which is necessarily adaptive to support changing external goals (Smallwood, Brown, Baird, & Schooler, 2012). While the specific role of the dlPFC in RC is unclear, neuroimaging work and behavioral studies on WM suggest that the dlPFC may play a role in both WL and DL reading processes, including support of word-to-text integration (Petten, Weckerly, Mclsaac, & Kutas, 1997; Stafura & Perfetti, 2014) and discourse construction/coherence (Coelho et al., 2012).
Interestingly, our covariate findings support the hypothesis that passage and word overlap regions have divergent, task-specific activation patterns (see Figures 4 and 5). RC ability only correlated with increased activation in critical areas during passage reading (not WL reading), and language/WM regions predicted by RC ability all fell within the passage and word overlap regions (see Figure 4). Consequently, stronger readers appear to elicit greater activation in language and executive overlap regions only during passage reading, despite the necessity of these regions in both word and passage reading. Conversely, word recognition ability correlated with WL phonological and semantic processing regions, including the left OT area, left MTG, and left IFG (see Figure 5). Interestingly, the left OT area and left MTG regions were also implicated in word reading ability in our connectivity findings (see Q2). These findings parallel behavioral studies on reading, which have found that RC ability correlates with a wider number of cognitive tasks than basic reading tasks do, including WM, inference, vocabulary, and sentence-level semantic processes (Oakhill et al., 2003; Sesma et al., 2009). Greater activation of portions of the DMN parallels behavioral findings that stronger readers have greater inferential processing ability (Ferstl et al., 2008; Locascio et al., 2010; Sesma et al., 2009).
The differential activation of these regions provide preliminary support of complex, task-specific activation patterns within overlap areas. In our next analyses, we sought to describe how these regions flexibly perform diverse functions within different levels of reading through connectivity analyses.
Question 2: Differential functional connectivity networks during word- and discourse processing
To examine networks that might underlie multi-tier RC deficits, we isolated regions whose mean activation overlapped during word and passage reading, and which have been implicated in WL and DL reading processes: (a) the pVWFA, a region previously found to critically contribute to reading through support of both orthographic and orthographic-semantic linking (b) IFG, MTG, and TP, all implicated in the frontal-temporal semantic network (henceforth referred to as language overlap regions; (Binder et al., 2009), and (c) the dlPFC, as prior studies suggest that WM, which is supported by the dlPFC, may play a role in both WL and DL functions. Our findings indicate that key language and WM regions that show shared activation in word and passage reading have different network correlations for these respective tasks. Importantly, this differentiation was predicted by reading ability.
Word Reading
WL correlations with language overlap regions and pVWFA
WL connectivity was characterized by coordination between semantic and orthographic processing regions. All three language overlap regions showed convergent correlations with a key region in visual word recognition, the left occipitotemporal area (extending to the pVWFA; see Figure 6). Similarly, the pVWFA seed showed coordination with the left MTG (overlapping with the left MTG seed), a region consistently implicated in word-level semantic storage and vocabulary processes (see Figure 7). Importantly, this pVWFA-to-MTG coupling was associated with WL reading ability (see Figure 10). This is consistent with previous studies which show that reading ability is positively associated with connectivity between pVWFA and regions in the language network during resting state (Koyama et al., 2011), and that typically developed readers show greater bottom-up communication from the fusiform gyrus to left MTG as compared to children with reading deficits during single word reading (Liu et al., 2010). Our findings suggest that WL processing and WL reading ability are characterized both by activation of semantic and orthographic regions, as well as greater coupling between semantic and orthographic processing networks. These findings are consistent with behavioral models of reading. According to the Lexical Quality Hypothesis, adequate word recognition requires building appropriate semantic, orthographic, and phonological representations of the words, and for these representations to appropriately converge into a unified understanding of the word (Perfetti, 2007). This convergence of semantic and orthographic processing streams is additionally supported by previous structural findings. Both the pVWFA seed and the pVWFA result region (from the language overlap results) map onto structural connectivity subdivisions of this region that have previously been suggested to support orthographic-semantic linking (Fan, Anderson, Davis, & Cutting, 2014). Specifically, Fan et al. (2014) found that anterior portions of the OT area are more structurally connected to semantic processing regions than posterior portions. Thus, neural correlates of word reading ability appear to map on to behavioral specifications of strong WL processing.
We were additionally interested in performing preliminary analysis to examine whether these results might be driven by functionally distinct subdivisions. The left IFG has historically been examined in the context of multifunctionality (Hagoort, 2005) versus subdivisions (Friederici, 2011). Interestingly, functional connectivity results from structural divisions of the IFG seed (left BA 44, 45, and 47) showed that both BA 44 and BA 47 correlated with distinct portions of the left pVWFA and general OT areas (see Supplemental Figure 1). In word reading, BA 44 is traditionally associated with phonological processing pathways, while BA 47 has greater associations with semantic pathways (Price, 2012; Friederici, 2011). BA 45 did not correlate with the left OT, but did show distinct correlations with primary sensory regions. While this analysis is still limited to large areas of tissue that are known to contain additional functional subdivisions (Amunts & Zilles, 2012), these findings demonstrate that WL connectivity findings from the L IFG seed are driven by distributed IFG subregions. Future analysis should examine the specific network properties of these subdivisions in the context of Passage and Word reading.
WL correlations with dlPFC
The dlPFC seed also showed correlations with an extended network that largely overlapped with the pVWFA network findings. These included correlations with primary sensorimotor regions, frontal regions (bilateral IFG/dlPFC), and the left MTG during WL processing (see Figure 9). Interestingly, the shared left MTG correlation from dlPFC and pVWFA networks also overlapped with our left MTG seed regions. Though the dlPFC correlation network was not predicted by WL reading ability, these coupled networks support previous assertions that the dLPFC and WM functions in general may play an important “top down” role in the maintenance of lexical information (Christopher et al., 2012). This includes the coordination of visual, orthographic, semantic and potential semantic embodiment information (Pulvermüller, 2013), though further study is needed to ascertain the specific directional relationships between these networks.
Word processing summary
Overall, connectivity findings for WL processing were highly consistent with the central role of the pVWFA in word recognition, and suggest that stronger basic readers not only have greater activation of word recognition areas (as found in our GLM results), but also have greater communication between these areas and other semantic and orthographic processing regions. Additionally, this semantic-orthographic pipeline appears to interact closely with the dlPFC.
Discourse Processing Networks
In contrast to the WL findings, we found that DL processing showed a strikingly different pattern of connectivity results for the language overlap regions, pVWFA, and most particularly the dlPFC. Notably, DL processing was characterized by independent, convergent positive correlations between all seed regions and the left AG (see Figures 6–8). The left AG is a heteromodal region that is implicated in a wide range of cognitive functions, including spatial cognition, the DMN, math processing, and semantics (Seghier, 2013). Within the context of language, the left AG has been extensively studied and consistently found to support global semantic/conceptual integration processes, including the integration of local semantic information into larger meaningful textual representations (Price, Bonner, Peelle, & Grossman, 2015; Seghier, 2013). Furthermore, a study by Hampson et al. (2006) found that correlations between Broca’s area and the left AG was predicted by reading ability in adults reading single sentences. As DL processing requires the coordinated effort to combinatorically integrate word-pair-, sentence-, and discourse-level units of meaning, in addition to maintaining previous units of information in WM, it is theoretically consistent that DL processing involves tighter coupling between the whole overlap network and the left AG.
DL correlations of language overlap regions and pVWFA
In addition to coupling with the left AG, DL processing was marked by greater coupling within the traditional left-hemisphere language network, including the language overlap regions and the pVWFA. Specifically, the left IFG, TP, and posterior MTG were correlated with each other in DL processing (see Table 7). These three regions are thought to form an executive semantic control network (Whitney et al., 2011), which processes local combinatorial semantic information and semantic inferences, as opposed to the more global processes of the left AG. Consequently, compared to the orthographic-semantic network found in WL processing, our results characterize DL processing with local-global semantic network interactions. This is consistent with neural models of language processing (Friederici, 2011), in which sub-sentence information is necessarily passed to (and informed by; Stafura & Perfetti, 2014) higher-level processing centers (left AG) in order to be integrated into a cohesive internal model (Whitney et al., 2009).
Supplemental examination of IFG subdivisions in DL processing showed that both BA 45 and BA 47 correlated with left AG in Passages compared to Words (see Supplemental Figure 1). BA 45 and 47 are part of the heteromodal granular layers of the frontal cortex (Hagoort, 2005), and are identified as part of semantic processing structural and functional pathways (Friederici, 2011). Interestingly, in conjunction with the WL analysis, these findings suggest that BA 47 alone exhibits flexible correlation patterns specifically with WL and DL “hub” regions, potentially reflecting its heterogeneous role in semantics (word- and discourse-level) and syntax (Price, 2012). This BA 47-specific connectivity pattern is also consistent with a study by Xiang et al. (2010) which showed that during resting state, ventral IFG (pars orbitalis) uniquely correlated with left AG and left OT areas compared to the rest of the IFG. These findings again demonstrate that DL results are driven by distributed, rather than focal, subdivisions within the left IFG.
Interestingly, compared to WL reading, the pVWFA was also found to be tightly coupled with both local (left TP and left MTG) and global (left AG) semantic processing nodes, as well as with the left SMG, a region associated with phonological processing (Price, 2012; see Figure 7). In the context of the Lexical Quality Hypothesis, this could suggest that DL processing requires more rigorous coordination between the phonological-orthographic-semantic nodes, as represented by pVWFA, left SMG, and local/global semantic regions (left MTG, left TP, left AG), respectively. However, these network connections were not found to be mediated by basic reading ability or RC ability, and further study is needed to tease apart the specific functional roles of this network.
DL correlations of dlPFC
Our results show that the dlPFC seed was not only correlated with the left AG in DL comprehension, but also that this correlation was positively associated with RC ability (see Figures 8 and 10). These findings therefore suggest that adolescent RC ability is marked by greater activation of and coordination between higher-order regions responsible for conceptual coherence functions (left AG; A. R. Price, Bonner, Peelle, & Grossman, 2015; Seghier, 2013) and top-down information maintenance/organization functions (left dlPFC; Ptak, 2012).
The dlPFC and its associated cognitive functions are hypothesized to support the integration, prediction, and organization of different types of incoming text information (Christopher et al., 2012; Fedorenko, 2014). Consequently, the WM and executive control behaviors associated with the dlPFC are of particular interest in the context of RC deficits. Executive functions related to dlPFC are independently associated with success in word reading and RC (Cain & Oakhill, 2006; Locascio et al., 2010; Oakhill & Cain, 2012). Additionally, readers with lower WM ability have shown decreased efficiency in local-global contextual dependence (Petten et al., 1997).
These findings have led to behavioral models of reading in which executive functions not only independently supports fluency and maintenance of conceptual information for WL and DL processing, respectively, but also supports the appropriate integration of WL and DL information (Cutting et. al, 2015). Within this framework, we would expect the dlPFC to mediate the relationship between WL and DL networks, and thus facilitate phonological, semantic, combinatorial semantic, and conceptual integration. While the methods used in this paper do not allow for causal interpretations, our supplemental analysis does suggest a role of the dlPFC in WL and DL integration (see Supplemental Figure 2). Specifically, examination of the interaction of dlPFC activation and left AG whole-brain connectivity (in Passage vs. Baseline) showed that one unit increase of dlPFC activation positively predicted coupling between the left AG and the left pVWFA—the two primary convergent nodes for DL and WL processing, respectively. Further, the relationship between left AG and OT areas is positively predicted by WM capacity. This suggests that the WM capabilities of dlPFC potentially facilitate greater communication between WL and DL networks. Future studies should explicitly examine WM and other executive function measurements and their predictions of network interactions, as well as apply causal modelling to examine directional relationships between the dlPFC, AG, and the language network.
In addition to the left AG, the left dlPFC was also found to coordinate with the left-lateralized DMN. These DMN nodes overlapped with our findings from the regions whose activation was predicted by RC ability. Previous studies have suggested that the dlPFC acts to guide DMN-related internal thought processes (Smallwood et al., 2012), and interruption of dlPFC through transcranial direct current stimulation has been found to disrupt the unification of DMN connectivity in resting state (Keeser et al., 2011; Peña-Gómez et al., 2012). In the context of expository text comprehension, the executive control capacity of the dlPFC may support the inferential processes of the DMN (Singer, 1997).
Discourse Processing Connectivity: Summary
Overall, our findings indicate that the language and WM overlap regions take on additional roles in the context of higher-level comprehension demands which support the integration of information into a cohesive, internal model. Such findings are consistent with what is known about distinctions between WL and DL processing: beyond WL processing, RC requires combinatorial semantic and syntactic processes that allow for phrase-level meaning construction (Friederici, 2011; Hagoort & Indefrey, 2014). These units then must be integrated into a cohesive global understanding of the text (Kendeou et al., 2014). In this vein, the left IFG, MTG, and in some cases the left TP, have each been shown to be involved in both semantic and syntactic unification processes. These processes include both prediction of upcoming information (forward processing), and integration of previous information (backward processing; Hagoort & Indefrey, 2014). Given the previous associations of the dlPFC in information maintenance and coherence, and the present findings that the dlPFC correlation with left AG is associated with RC ability, it is possible that the dlPFC plays an important role in these integrative processes. However, additional analysis is needed in order to make assertions of causal roles within these networks.
Conclusions
Our findings indicate that word and passage reading recruit activation in overlapping regions, but these areas form task-specific networks within and beyond the language network. Specifically, our functional connectivity analyses indicate that overlap areas in the language network exhibit multi-functional, task-specific correlations, and that these correlations are predicted by WL reading and RC ability. Word reading is characterized by connections between lexico-semantic regions and orthographic processing regions, and these orthographic-semantic connections are predicted by word decoding ability. Passage reading not only involves these WL processing networks, but additional communication between the same overlap areas and the global integration processes in the left AG to support DL processes. RC ability is predictive of coupling between higher-order information maintenance and meaning coherence regions. Consequently, stronger comprehenders appear to not only have greater activation of language/WM overlap regions and broader executive regions, but to also have greater communication between regions associate with executive functions.
These findings point to flexible network processes within reading “hub” regions. The interaction between these networks and RC ability encourage additional exploration of overlap regions in naturalistic reading environments. More generally, our findings highlight the fact that shared regional activity is not necessarily indicative of shared functions, even within the same cognitive domain. While it has long been known that brain regions perform multiple cognitive functions, our results suggest that connectivity may be critical for truly dissecting differences, even within similar tasks where one is presumed to be controlling for some aspect of the other.
The current study has a few limitations as well as areas that should be more extensively examined in future studies. First, as there is some evidence that OT regions respond to visual properties, it is possible that Words vs. Symbols pVWFA connectivity results were confounded by lower-level visual processes driven by visual discrepancies between the two stimuli types. However, the fact that all reported WL connectivity findings are replicated in Words alone, and are also consistent with functional and structural associations from other literature, suggests that the effect of visual characteristic differences on the current results is likely minimal. Secondly, regions of interest in the current study were limited to large brain areas; to further the current exploration of multifunctionality in reading, future studies should examine network patterns from smaller subdivisions of overlap regions. The current study also encourages further investigation of network properties in young readers, including the use of interventional paradigms to examine how RC interventions mediate connectivity patterns in struggling reader populations. The directional roles of these nodes can also be explored with causal modeling of connectivity data. Additional work should examine how these network connections are influenced by text genre, as expository text specifically places increased demand on WM capacity and semantic processing, and decreased demand on social cognition. Through these examinations, neuroimaging techniques can be used to set the groundwork for neurobiologically-informed RC interventions. Such knowledge not only has the potential to improve clinical and educational approaches to developmental reading processes, but also to contribute to our general understanding of basic language processes.
Supplementary Material
Research Highlights.
We examined the relationship of word-level and discourse-level neural processing in a range of adolescent readers.
We isolated regions of overlapping activation for word and passage reading, and observed differential functional connectivity networks of these regions for word-level and discourse-level processing.
Importantly, we found that word reading and reading comprehension ability predicted the strength of differential functional connectivity patterns of passage and word overlap regions.
Findings not only have significant implications for dynamic neural processes during reading, but more generally emphasize that shared regional activity across similar cognitive tasks is not necessarily indicative of shared function.
Acknowledgments
This research was supported by the following funding sources: RO1 HD044073, P30 HD015052, 5RO1 HD067254, U54 HD083211, R24 HD075460, and CTSA RR024975
References
- Amunts K, Zilles K. Architecture and organizational principles of Broca’s region. Trends in Cognitive Sciences. 2012;16(8):418–426. doi: 10.1016/j.tics.2012.06.005. http://doi.org/10.1016/j.tics.2012.06.005. [DOI] [PubMed] [Google Scholar]
- Baretta L, Tomitch LMB, MacNair N, Lim VK, Waldie KE. Inference making while reading narrative and expository texts: An ERP study. Psychology & Neuroscience. 2009;2(2):137–145. [Google Scholar]
- Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage. 2007;37(1):90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman RA, Nir-sagiv B. Comparing Narrative and Expository Text Construction Across Adolescence: A Developmental Paradox. 2007 Retrieved from http://www.tandfonline.com/doi/full/10.1080/01638530709336894#tabModule. [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cerebral Cortex (New York, N.Y.?: 1991) 2009;19(12):2767–2796. doi: 10.1093/cercor/bhp055. http://doi.org/10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitan T, Booth JR, Choy J, Burman DD, Gitelman DR, Mesulam M-M. Shifts of effective connectivity within a language network during rhyming and spelling. The Journal of Neuroscience?: The Official Journal of the Society for Neuroscience. 2005;25(22):5397–5403. doi: 10.1523/JNEUROSCI.0864-05.2005. http://doi.org/10.1523/JNEUROSCI.0864-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Cain K, Oakhill J. Profiles of children with specific reading comprehension difficulties. The British Journal of Educational Psychology. 2006;76(Pt 4):683–696. doi: 10.1348/000709905X67610. [DOI] [PubMed] [Google Scholar]
- Cappa SF. Imaging semantics and syntax. NeuroImage. 2012;61(2):427–431. doi: 10.1016/j.neuroimage.2011.10.006. [DOI] [PubMed] [Google Scholar]
- Christopher ME, Miyake A, Keenan JM, Pennington B, DeFries JC, Wadsworth SJ, Olson RK. Predicting word reading and comprehension with executive function and speed measures across development: a latent variable analysis. Journal of Experimental Psychology. General. 2012;141(3):470–488. doi: 10.1037/a0027375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho C, Lê K, Mozeiko J, Krueger F, Grafman J. Discourse production following injury to the dorsolateral prefrontal cortex. Neuropsychologia. 2012;50(14):3564–3572. doi: 10.1016/j.neuropsychologia.2012.09.005. [DOI] [PubMed] [Google Scholar]
- Cole MW, Reynolds JR, Power JD, Repovs G, Anticevic A, Braver TS. Multi-task connectivity reveals flexible hubs for adaptive task control. Nature Neuroscience. 2013;16(9):1348–1355. doi: 10.1038/nn.3470. http://doi.org/10.1038/nn.3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutting LE, Bailey SK, Barquero LA, Aboud K. Neurobiological bases of word recognition and reading comprehension. In: Connor CM, McCardle P, editors. Advances in Reading Intervention: Research to Practice to Research. Brookes Publishing Co; 2015. [Google Scholar]
- Cutting LE, Materek A, Cole CAS, Levine TM, Mahone EM. Effects of fluency, oral language, and executive function on reading comprehension performance. Annals of Dyslexia. 2009;59(1):34–54. doi: 10.1007/s11881-009-0022-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijk TA, Van, Kintsch W. Strategies of Discourse Comprehension. New York: Academic Press; 1983. [Google Scholar]
- Eason SH, Goldberg LF, Young KM, Geist MC, Cutting LE. Reader-text interactions: How differential text and question types influence cognitive skills needed for reading comprehension. Journal of Educational Psychology. 2012;104(3):515–528. doi: 10.1037/a0027182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadiga L, Craighero L, Destro MF, Finos L, Cotillon-Williams N, Smith AT, Castiello U. Language in shadow. Social Neuroscience. 2006;1(2):77–89. doi: 10.1080/17470910600976430. http://doi.org/10.1080/17470910600976430. [DOI] [PubMed] [Google Scholar]
- Fan Q, Anderson AW, Davis N, Cutting LE. Structural connectivity patterns associated with the putative visual word form area and children’s reading ability. Brain Research. 2014;1586:118–129. doi: 10.1016/j.brainres.2014.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E. The role of domain-general cognitive control in language comprehension. Frontiers in Psychology. 2014;5:335. doi: 10.3389/fpsyg.2014.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferstl EC, Neumann J, Bogler C, von Cramon DY. The extended language network: a meta-analysis of neuroimaging studies on text comprehension. Human Brain Mapping. 2008;29(5):581–593. doi: 10.1002/hbm.20422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frackowiak RSJ, Friston KJ, Frith CD, Dolan RJ, Mazziotta JC. Human Brain Function. Academic Press; USA: 1997. [Google Scholar]
- Friederici AD. The brain basis of language processing: from structure to function. Physiological Reviews. 2011;91(4):1357–1392. doi: 10.1152/physrev.00006.2011. [DOI] [PubMed] [Google Scholar]
- Hagoort P. On Broca, brain, and binding: a new framework. Trends in Cognitive Sciences. 2005;9(9):416–423. doi: 10.1016/j.tics.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Hagoort P, Indefrey P. The neurobiology of language beyond single words. Annual Review of Neuroscience. 2014;37:347–362. doi: 10.1146/annurev-neuro-071013-013847. [DOI] [PubMed] [Google Scholar]
- Hampson M, Tokoglu F, Sun Z, Schafer RJ, Skudlarski P, Gore JC, Constable RT. Connectivity-behavior analysis reveals that functional connectivity between left BA39 and Broca’s area varies with reading ability. NeuroImage. 2006;31(2):513–519. doi: 10.1016/j.neuroimage.2005.12.040. http://doi.org/10.1016/j.neuroimage.2005.12.040. [DOI] [PubMed] [Google Scholar]
- Hoover WA, Gough PB. The simple view of reading. Reading and Writing. 1990;2(2):127–160. http://doi.org/10.1007/BF00401799. [Google Scholar]
- Humphries C, Binder JR, Medler DA, Liebenthal E. Time course of semantic processes during sentence comprehension: an fMRI study. NeuroImage. 2007;36(3):924–932. doi: 10.1016/j.neuroimage.2007.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferies E. The neural basis of semantic cognition: converging evidence from neuropsychology, neuroimaging and TMS. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior. 2013;49(3):611–625. doi: 10.1016/j.cortex.2012.10.008. [DOI] [PubMed] [Google Scholar]
- Keeser D, Meindl T, Bor J, Palm U, Pogarell O, Mulert C, Padberg F. Prefrontal Transcranial Direct Current Stimulation Changes Connectivity of Resting-State Networks during fMRI. Journal of Neuroscience. 2011;31(43):15284–15293. doi: 10.1523/JNEUROSCI.0542-11.2011. http://doi.org/10.1523/JNEUROSCI.0542-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendeou P, van den Broek P, Helder A, Karlsson J. A Cognitive View of Reading Comprehension: Implications for Reading Difficulties. Learning Disabilities Research & Practice. 2014;29(1):10–16. [Google Scholar]
- Kovelman I, Norton ES, Christodoulou JA, Gaab N, Lieberman DA, Triantafyllou C, Gabrieli JDE. Brain basis of phonological awareness for spoken language in children and its disruption in dyslexia. Cerebral Cortex (New York, N.Y.?: 1991) 2012;22(4):754–764. doi: 10.1093/cercor/bhr094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama MS, Di Martino A, Zuo X-N, Kelly C, Mennes M, Jutagir DR, Milham MP. Resting-state functional connectivity indexes reading competence in children and adults. Journal of Neuroscience. 2011;31(23):8617–8624. doi: 10.1523/JNEUROSCI.4865-10.2011. http://doi.org/10.1523/JNEUROSCI.4865-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Summerln JL, Rainey L, Freitas CS, Fox PT. he Talairach Daemon, a database server for Talairach Atlas Labels. NeuroImage. 1997;5:S633. [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT. Automated Talairach atlas labels for functional brain mapping. Hum. Brain Mapp. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Vira A, Friedman E, Minas J, Bolger D, Bitan T, Booth J. Children with reading disability show brain differences in effective connectivity for visual, but not auditory word comprehension. PloS One. 2010;5(10):e13492. doi: 10.1371/journal.pone.0013492. http://doi.org/10.1371/journal.pone.0013492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locascio G, Mahone EM, Eason SH, Cutting LE. Executive dysfunction among children with reading comprehension deficits. Journal of Learning Disabilities. 2010;43(5):441–454. doi: 10.1177/0022219409355476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGinitie WH, MacGinitie RK, Maria K, Dreyer LG. Gates-MacGinitie Reading Tests. 4th. Itasca, IL: Riverside; 2000. [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fmri data sets. NeuroImage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. (WFU Pickatlas, version 2.5.2) [DOI] [PubMed] [Google Scholar]
- Mar RA. The Neural Bases of Social Cognition and Story Comprehension. 2011 doi: 10.1146/annurev-psych-120709-145406. Retrieved March 10, 2014, from http://www.annualreviews.org/doi/pdf/10.1146/annurev-psych-120709-145406. [DOI] [PubMed] [Google Scholar]
- McCandliss BD, Cohen L, Dehaene S. The visual word form area: expertise for reading in the fusiform gyrus. Trends in Cognitive Sciences. 2003;7(7):293–299. doi: 10.1016/s1364-6613(03)00134-7. [DOI] [PubMed] [Google Scholar]
- McNamara DS, Louwerse MM, Cai Z, Graesser A. Coh-Metrix version 1.4. 2005 Jan 1; Retrieved 2012, from http//:cohmetrix.memphis.edu. [Google Scholar]
- Nation K, Adams JW, Bowyer-Crane Ca, Snowling MJ. Working memory deficits in poor comprehenders reflect underlying language impairments. Journal of Experimental Child Psychology. 1999;73(2):139–158. doi: 10.1006/jecp.1999.2498. [DOI] [PubMed] [Google Scholar]
- Nation K, Snowling MJ, Clarke P. Dissecting the relationship between language skills and learning to read: Semantic and phonological contributions to new vocabulary learning in children with poor reading comprehension†. International Journal of Speech-Language Pathology. 2007;9(2):131–139. [Google Scholar]
- Newman SD, Malaia E, Seo R, Cheng H. The effect of individual differences in working memory capacity on sentence comprehension: an FMRI study. Brain Topography. 2013;26(3):458–467. doi: 10.1007/s10548-012-0264-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakhill JV, Cain K, Bryant PE. The dissociation of word reading and text comprehension: Evidence from component skills. Language and Cognitive Processes. 2003;18(4):443–468. [Google Scholar]
- Oakhill JV, Cain K. The Precursors of Reading Ability in Young Readers: Evidence From a Four-Year Longitudinal Study. Scientific Studies of Reading. 2012;16(2):91–121. [Google Scholar]
- Patterson K, Nestor PJ, Rogers TT. Where do you know what you know? The representation of semantic knowledge in the human brain. Nature Reviews. Neuroscience. 2007;8(12):976–987. doi: 10.1038/nrn2277. [DOI] [PubMed] [Google Scholar]
- Peña-Gómez C, Sala-Lonch R, Junqué C, Clemente IC, Vidal D, Bargalló N, Bartrés-Faz D. Modulation of large-scale brain networks by transcranial direct current stimulation evidenced by resting-state functional MRI. Brain Stimulation. 2012;5(3):252–263. doi: 10.1016/j.brs.2011.08.006. http://doi.org/10.1016/j.brs.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfetti C. Reading Ability: Lexical Quality to Comprehension. Scientific Studies of Reading. 2007;11(4):357–383. [Google Scholar]
- Petten C Van, Weckerly J, Mclsaac HK, Kutas M. WORKING MEMORY CAPACITY DISSOCIATES LEXICAL AND SENTENTIAL CONTEXT EFFECTS. 1997;8(3) [Google Scholar]
- Power JD, Schlaggar BL, Lessov-Schlaggar CN, Petersen SE. Evidence for hubs in human functional brain networks. Neuron. 2013;79(4):798–813. doi: 10.1016/j.neuron.2013.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price AR, Bonner MF, Peelle JE, Grossman M. Converging evidence for the neuroanatomic basis of combinatorial semantics in the angular gyrus. The Journal of Neuroscience?: The Official Journal of the Society for Neuroscience. 2015;35(7):3276–3284. doi: 10.1523/JNEUROSCI.3446-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ. A review and synthesis of the first 20 years of PET and fMRI studies of heard speech, spoken language and reading. NeuroImage. 2012;62(2):816–847. doi: 10.1016/j.neuroimage.2012.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptak R. The frontoparietal attention network of the human brain: action, saliency, and a priority map of the environment. The Neuroscientist?: A Review Journal Bringing Neurobiology, Neurology and Psychiatry. 2012;18(5):502–515. doi: 10.1177/1073858411409051. http://doi.org/10.1177/1073858411409051. [DOI] [PubMed] [Google Scholar]
- Pugh KR, Mencl WE, Jenner AR, Katz L, Frost SJ, Lee JR, Shaywitz BA. Neurobiological studies of reading and reading disability. Journal of Communication Disorders. 2001;34(6):479–492. doi: 10.1016/s0021-9924(01)00060-0. [DOI] [PubMed] [Google Scholar]
- Pulvermüller F. Semantic embodiment, disembodiment or misembodiment? In search of meaning in modules and neuron circuits. Brain and Language. 2013;127(1):86–103. doi: 10.1016/j.bandl.2013.05.015. http://doi.org/10.1016/j.bandl.2013.05.015. [DOI] [PubMed] [Google Scholar]
- Rayner K. Eye movements and the perceptual span in beginning and skilled readers. Journal of Experimental Child Psychology. 1986;41:211–236. doi: 10.1016/0022-0965(86)90037-8. [DOI] [PubMed] [Google Scholar]
- Richlan F. Developmental dyslexia: dysfunction of a left hemisphere reading network. Frontiers in Human Neuroscience. 2012;6:120. doi: 10.3389/fnhum.2012.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richlan F, Kronbichler M, Wimmer H. Functional abnormalities in the dyslexic brain: a quantitative meta-analysis of neuroimaging studies. Human Brain Mapping. 2009;30(10):3299–3308. doi: 10.1002/hbm.20752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimrodt SL, Clements-Stephens AM, Pugh KR, Courtney SM, Gaur P, Pekar JJ, Cutting LE. Functional MRI of sentence comprehension in children with dyslexia: beyond word recognition. Cerebral Cortex (New York, N.Y.?: 1991) 2009;19(2):402–413. doi: 10.1093/cercor/bhn092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaggar BL, McCandliss BD. Development of neural systems for reading. Annual Review of Neuroscience. 2007;30:475–503. doi: 10.1146/annurev.neuro.28.061604.135645. [DOI] [PubMed] [Google Scholar]
- Seghier ML. The angular gyrus: multiple functions and multiple subdivisions. The Neuroscientist?: A Review Journal Bringing Neurobiology, Neurology and Psychiatry. 2013;19(1):43–61. doi: 10.1177/1073858412440596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesma HW, Mahone EM, Levine T, Eason SH, Cutting LE. The Contribution of Executive Skills to Reading Comprehension. Child Neuropsychology. 2009;15(3):232–246. doi: 10.1080/09297040802220029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer M, Harkness D, Steward ST. Constructing inferences in expository text comprehension. Discourse Processes. 1997;24(2–3):199–228. doi: http://dx.doi.org/10.1080/01638539709545013. [Google Scholar]
- Smallwood J, Brown K, Baird B, Schooler JW. Cooperation between the default mode network and the frontal-parietal network in the production of an internal train of thought. Brain Research. 2012;1428:60–70. doi: 10.1016/j.brainres.2011.03.072. http://doi.org/10.1016/j.brainres.2011.03.072. [DOI] [PubMed] [Google Scholar]
- Spencer M, Quinn JM, Wagner RK. Specific Reading Comprehension Disability: Major Problem, Myth, or Misnomer? Learning Disabilities Research & Practice?: A Publication of the Division for Learning Disabilities, Council for Exceptional Children. 2014;29(1):3–9. doi: 10.1111/ldrp.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafura JZ, Perfetti CA. Word-to-text integration: Message level and lexical level influences in ERPs. Neuropsychologia. 2014;64C:41–53. doi: 10.1016/j.neuropsychologia.2014.09.012. http://doi.org/10.1016/j.neuropsychologia.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stothard SE. Reading comprehension difficulties in children The role of language comprehension and working memory skills. 1992:245–256. [Google Scholar]
- Swanson HL, Cochran KF, Ewers Ca. Working memory in skilled and less skilled readers. Journal of Abnormal Child Psychology. 1989;17(2):145–156. doi: 10.1007/BF00913790. http://doi.org/10.1007/BF00913790. [DOI] [PubMed] [Google Scholar]
- Tsapkini K, Frangakis CE, Hillis AE. The function of the left anterior temporal pole: evidence from acute stroke and infarct volume. Brain?: A Journal of Neurology. 2011;134(Pt 10):3094–3105. doi: 10.1093/brain/awr050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U. S. Department of Education, Institute of Education Sciences, National Center for Education Statistics. National Assessment of Educational Progress. 2013 [Google Scholar]
- Vandermosten M, Boets B, Wouters J, Ghesquiere P. A qualitative and quantitative review of diffusion tensor imaging studies in reading and dyslexia. Neuroscience and Biobehavioral Reviews. 2012;36(6):1532–1552. doi: 10.1016/j.neubiorev.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Visser M, Jefferies E, Embleton KV, Lambon Ralph MA. Both the middle temporal gyrus and the ventral anterior temporal area are crucial for multimodal semantic processing: distortion-corrected fMRI evidence for a double gradient of information convergence in the temporal lobes. Journal of Cognitive Neuroscience. 2012;24(8):1766–1778. doi: 10.1162/jocn_a_00244. [DOI] [PubMed] [Google Scholar]
- Vogel AC, Petersen SE, Schlaggar BL. The VWFA: it's not just for words anymore. Frontiers in Human Neuroscience. 2014;8:88. doi: 10.3389/fnhum.2014.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreivated Scale of Intelligence- Second Addition. San Antonio, TX: NCS Pearson; 2011. [Google Scholar]
- Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connectivity. 2012;2(3):125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- Whitney C, Huber W, Klann J, Weis S, Krach S, Kircher T. Neural correlates of narrative shifts during auditory story comprehension. NeuroImage. 2009;47(1):360–366. doi: 10.1016/j.neuroimage.2009.04.037. [DOI] [PubMed] [Google Scholar]
- Whitney C, Kirk M, O’Sullivan J, Lambon Ralph MA, Jefferies E. The neural organization of semantic control: TMS evidence for a distributed network in left inferior frontal and posterior middle temporal gyrus. Cerebral Cortex (New York, N.Y.?: 1991) 2011;21(5):1066–1075. doi: 10.1093/cercor/bhq180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock RW. Woodcock reading mastery tests – Revised/Normative update. Circle Pines, MN: American Guidance Service; 1998. [Google Scholar]
- Xiang H-D, Fonteijn HM, Norris DG, Hagoort P. Topographical Functional Connectivity Pattern in the Perisylvian Language Networks. Cerebral Cortex. 2010;20(3):549–560. doi: 10.1093/cercor/bhp119. http://doi.org/10.1093/cercor/bhp119. [DOI] [PubMed] [Google Scholar]
- Yarkoni T, Poldrack R, Nichols T, Van Essen D, Wager T. NeuroSynth: a new platform for large-scale automated synthesis of human functional neuroimaging data. Front. Neuroinform; Conference Abstract: 4th INCF Congress of Neuroinformatics; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.