Figure 2. The progression of stages from the M1 to M2 phenotypes can be controlled at various time points through different strategies.
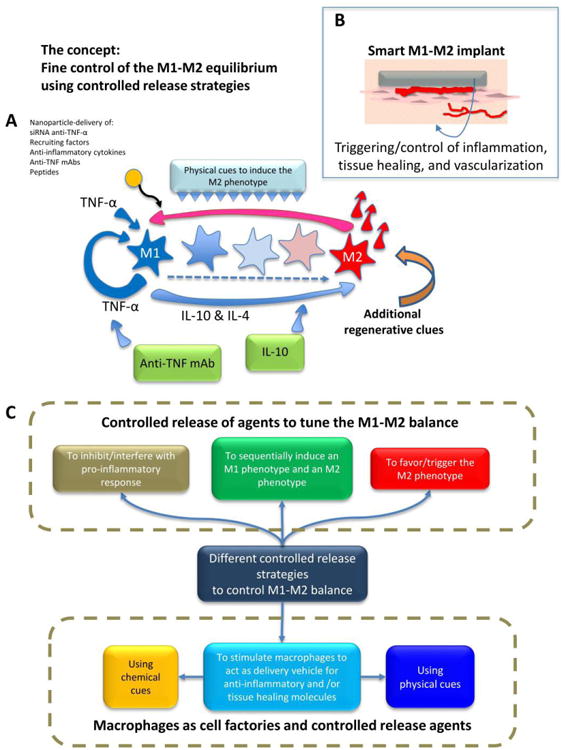
(A) A simplified representation of the signaling network relevant to inflammation and healing after implantation of a scaffold. The main actors in the network are represented: TNFα, IL-4, and IL-10. M1-M2 polarization can be controlled or disrupted by the controlled release of different molecules that interfere with pro-inflammatory cytokines (e.g., release of anti-TNFα monoclonal antibodies (mAbs) or proteins) or amplify anti-inflammatory stimuli (e.g., release of IL-10). Micro- and nanoparticles loaded with different chemical agents (e.g., siRNA, DNA, anti-TNFα mAbs, IL-10 and other cytokines, etc.) have been used as controlled-release agents to modulate the M1-M2 balance. (B) The ultimate aspiration would be to design and fabricate “smart tissue engineering scaffolds” with controlled release potential that would regulate the M1-M2 balance (and therefore the healing, tissue repair, and vascularization processes). While the M1 macrophage phenotype is needed immediately after injury, and inflammation is an important and required component of the proper response of the human body to injury, a timely shift towards the M2 phenotype is required to complete the healing/tissue regeneration process. (C) The different controlled release strategies reviewed here that aim to tune the M1-M2 equilibrium.
