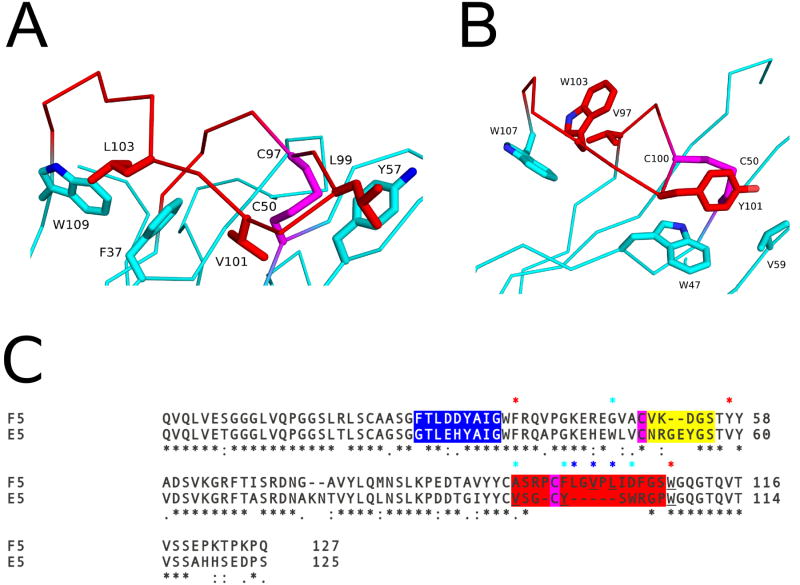
Figure 6. Key residues influencing CDR3 conformation.
The Cα–traces of (A) VHH F5 and (B) VHH E5. The VHHs are colored cyan with their CDR3 elements colored red. Key residues forming hydrophobic interactions are drawn as sticks and color coordinated to their respective main chain color. The disulfide bond between residues Cys50-Cys97 in VHH F5, and Cys50-Cys100 in E5, are shown in stick representation and colored magenta. (C) Sequence alignment of F5 and E5. CDR1, 2, and 3 are highlighted with blue, yellow, and red, respectively. Cysteines forming the disulfide between the CDR3 and FR residues are in magenta. Sequence positions of residues involved in the hydrophobic interactions between the CDR3 and FR residues in both F5 and E5 are highlighted with red asterisks above the sequence. Residues participating in hydrophobic interactions for F5 alone are highlighted with a blue asterisk and E5 alone with cyan asterisk above the sequence. Black asterisks below the sequence denote sequence identity.